Figure 1. Metabolism of Granulopoiesis.
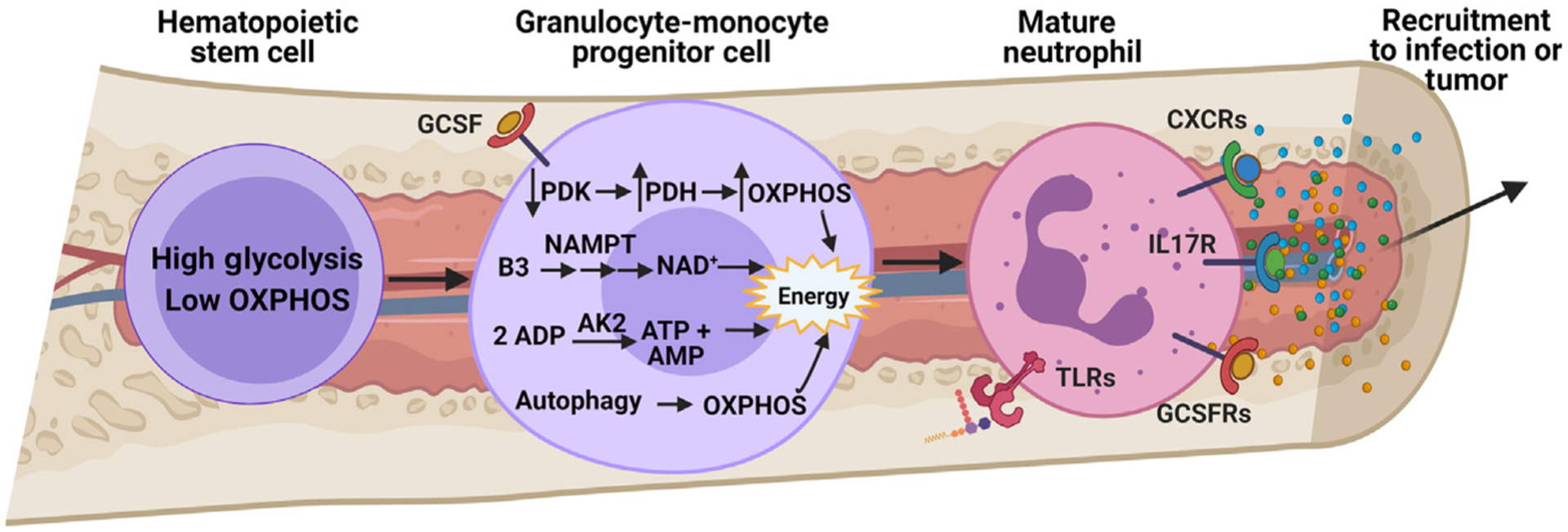
Granulopoiesis is a highly regulated differentiation process, leading to the generation of mature neutrophils from hematopoietic stem cells [4]. Granulocyte colony-stimulating factor (G-CSF) is the major cytokine promoting neutrophil differentiation in the bone marrow and also regulates salvage NAD+ synthesis through upregulation of nicotinamide phosphoribosyltransferase (NAMPT) [12]. This increase in NAD+ production coincides with engagement of oxidative phosphorylation (OXPHOS) as a result of increased oxygen availability. Improved OXPHOS function also occurs due to suppression of pyruvate dehydrogenase kinase (PDK), leading to the conversion of pyruvate to acetyl-CoA by pyruvate dehydrogenase (PDH) [9]. Regulation of adenylate nucleotides (AMP, ADP, and ATP) in the mitochondria by adenylate kinase-2 (AK2) is also necessary for proper granulopoiesis, wherein mutations in the gene encoding AK2 lead to reticular dysgenesis, a rare genetic disorder resulting in severe combined immunodeficiency [10]. Autophagy also supports mitochondrial respiration during granulopoiesis [14]. Emergency granulopoiesis by pathogen-derived byproducts also influences mature neutrophil metabolism through Toll-like receptors (TLRs) to engage the pentose phosphate pathway (PPP) and prime neutrophils for an oxidative burst once they reach the site of infection. In a similar fashion, tumor microenvironment (TME)-derived cytokines, such as G-CSF, chemokine (C-X-C motif) ligand 1/2 (CXCL1/2), or interleukin 17 (IL-17), can not only skew differentiation of hematopoietic cells toward granulocyte precursors and mature neutrophils [89], but also promote tissue-specific recruitment to support tumor progression and metastasis [90–92]. More research is needed to understand whether these TME-derived cytokines manipulate granulopoiesis-related metabolism or metabolically prime mature neutrophils toward an N2, protumor phenotype even before reaching a primary tumor or metastatic sites. Abbreviation: GCSFR, granulocyte colony-stimulating factor receptor.
