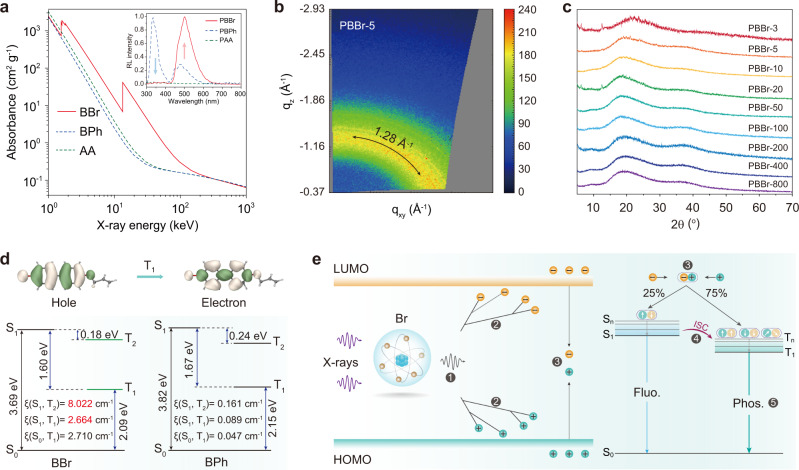
Fig. 3. Mechanism of phosphorescent scintillation from organic copolymers under X-ray irradiation.
a X-ray absorption spectra of the BBr, BPh, and AA monomers. The insert shows the normalized radioluminescence (RL) intensity of the PBBr, PBBh, and PAA polymers at a dose rate of 278 μGy s−1. b WAXS pattern of PBBr-5 polymer film. c PXRD patterns of the copolymers from PBBr-3 to PBBr-800. d Natural transition orbitals (NTOs) for the lowest triplet state of BBr, and calculated excitation energies and spin-orbit coupling (SOC) constants (ξ) of the BBr and BPh monomers. e Proposed mechanism of radioluminescence for amorphous copolymers. After X-ray irradiation, the electron in the inner shells is excited by high-energy X-ray photons and ejected out of the Br atom. Then, the high-energy electrons generate lots of secondary electrons by interacting with other atoms in the polymer. The generated electrons and holes are rapidly thermally dissipated and gradually accumulate at the lowest unoccupied molecular orbital (LUMO) and highest occupied molecular orbital (HOMO) of the organic phosphors, respectively. Eventually, the electrons and holes recombine to form excited states, generating singlet and triplet excitons in a ratio of 1:3, which produces fluorescence (Fluo.) and phosphorescence (Phos.) via the radiative decay processes, respectively.