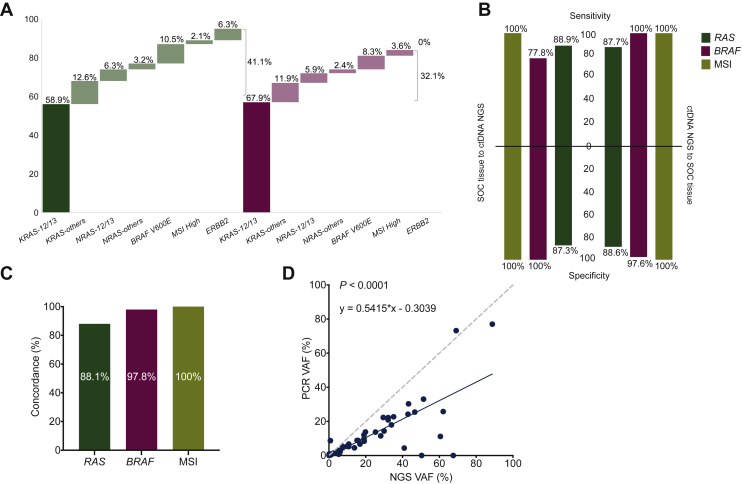
Figure 3.
Biomarker identity and concordance between comprehensive ctDNA and SOC tissue testing. (A) Biomarkers identified by comprehensive ctDNA and SOC tissue testing. (B) Clinical performance summary statistics for each test method using the other as the comparator for each biomarker. BRAF is BRAF V600E. (C) Concordance between test methods per biomarker. (D) Correlation between variant allelic fractions (VAFs) as determined by comprehensive ctDNA NGS and limited ctDNA PCR testing for KRAS.ctDNA, circulating tumor DNA; NGS, next-generation sequencing; SOC, standard of care.