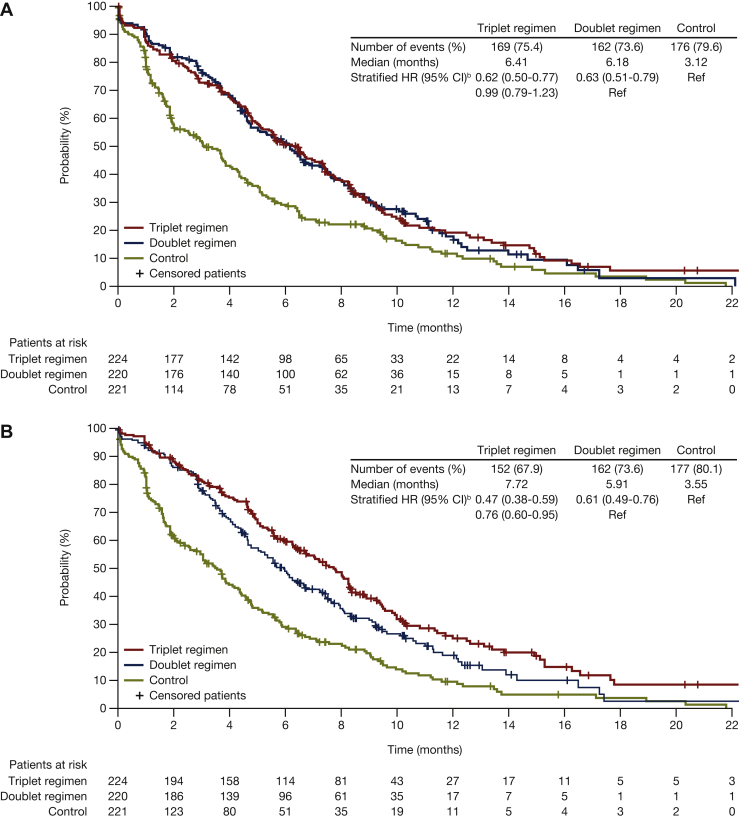
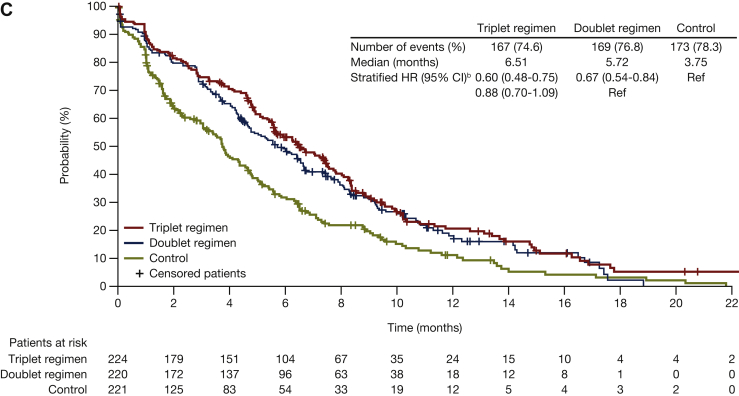
Figure 3.
Median time to definitive deteriorationa,bin EORTC for encorafenib plus cetuximab with or without binimetinib compared with control: (A) Physical functioning; (B) emotional functioning; (C) social functioning. Probability (%) of median time to definitive 10% deterioration in EORTC global health status subscales (A) physical functioning, (B) emotional functioning, and (C) social functioning for patients treated with encorafenib plus cetuximab with or without binimetinib (triplet and doublet, respectively) compared with the control. CI, confidence interval; HR, hazard ratio; EORTC, European Organization for Research and Treatment of Cancer Quality of Life Questionnaire Core 30.aDefinitive deterioration is at least 10% worsening relative to baseline with no later improvement above this threshold, or death due to any cause. bStratified by Eastern Cooperative Oncology Group performance status, source of cetuximab, and prior irinotecan use at randomization.