Abstract
Autoimmune systemic diseases (ASD) show impaired immunogenicity to COVID-19 vaccines. Our prospective observational multicenter study aimed at evaluating the seroconversion elicited by COVID-19 vaccine over the entire vaccination cycle including the booster dose.
Among 478 unselected ASD patients originally evaluated at the end of the first vaccination cycle (time 1), 344 individuals were re-evaluated after a 6-month period (time 2), and 244 after the booster vaccine dose (time 3). The immunogenicity of mRNA COVID-19 vaccines (BNT162b2 and mRNA-1273) was assessed by measuring serum IgG-neutralizing antibody (NAb) on samples obtained at the three time points in both patients and 502 age-matched controls.
In the 244 ASD group that received booster vaccine and monitored over the entire follow-up, the mean serum NAb levels (time 1, 2, and 3: 696.8 ± 52.68, 370.8 ± 41.92, and 1527 ± 74.16SD BAU/mL, respectively; p < 0.0001) were constantly lower compared to controls (p < 0.0001), but they significantly increased after the booster dose compared to the first two measurements (p < 0.0001). The percentage of patients with absent/suboptimal response to vaccine significantly decreased after the booster dose compared to the first and second evaluations (time 1, 2, and 3: from 28.2% to 46.3%, and to 7.8%, respectively; p < 0.0001). Of note, the percentage of patients with absent/suboptimal response after the booster dose was significantly higher compared to controls (19/244, 7.8% vs 1/502, 0.2%; p < 0.0001). Similarly, treatment with immune-modifiers increased the percentage of patients exhibiting absent/suboptimal response (16/122, 13.1% vs 3/122, 2.46%; p = 0.0031). Overall, the above findings indicate the usefulness of booster vaccine administration in ASD patients. Moreover, the persistence of a significantly higher percentage of individuals without effective seroconversion (7.8%), even after the booster dose, warrants for careful monitoring of NAb levels in all ASD patients to identify those with increased risk of infection. In this particularly frail patients’ setting, tailored vaccination and/or therapeutic strategy are highly advisable.
Keywords: Autoimmune systemic diseases, COVID-19 vaccine, Neutralizing antibodies, Systemic sclerosis, Rheumatoid arthritis, Systemic lupus, Cryoglobulinemic vasculitis, Systemic vasculitis, Booster vaccine
Abbreviations: autoimmune systemic diseases (ASD), rheumatoid factor (RF); anti-citrullinated protein antibodies (ACPA), rheumatoid arthritis (RA); systemic lupus erythematosus (SLE), systemic sclerosis (SSc); cryoglobulinemic vasculitis (CV), neutralizing antibody (NAb); World Health Organization (WHO), Binding Antibody Units (BAU)
1. Introduction
Patients with autoimmune systemic diseases (ASDs) show increased susceptibility to COVID-19 compared to the general population, possibly due to immune system alterations and/or ongoing treatment with immune-suppressants [[1], [2], [3], [4], [5]]. In addition, ASD patients with older age and/or pre-existing ASD-related interstitial lung involvement have more severe symptomology, leading to increased need for hospitalization and COVID-19-related death rates [2,[6], [7], [8]]. At the beginning of the pandemic, the stringent lockdown measures and the broad use of telemedicine limited the risk of developing COVID-19 in several disease settings, including the ASD population. Since the early 2021, the course of the pandemic was dramatically changed by the mass vaccination campaign. Importantly, the frail patients’ populations, such as ASD patients, were prioritized in several countries, including Italy (https://www.trovanorme.salute.gov.it/norme/renderPdf.spring?seriegu=SG&datagu=24/03/2021&redaz=21A01802&artp=1&art=1&subart=1&subart1=10&vers=1&prog=002) [9]. Overall, COVID-19 vaccines in ASD patients revealed good safety profile and immunogenicity [[10], [11], [12], [13]], with some limitations in patient receiving immune-modifier therapy, mainly rituximab (RTX), high glucocorticoid dosages, in the elderly and in particular ASD subsets [[11], [12], [13], [14]]. In February 2021, the COVID-19 & Italian ASD Study Group organized a prospective multicenter observational study aimed at evaluating the safety and efficacy (i.e., the ability to prevent symptomatic SARS-CoV-2 infection) of COVID-19 vaccines, as well as the possible negative effects of immune-suppressants on vaccine immunogenicity in ASD patients. The first vaccination cycle showed good safety profile in the ASD population. However, serum levels of IgG-neutralizing antibodies (NAb) were lower in the whole ASD series compared to the general population [15]. This percentage was particularly elevated in patients with ASD-related interstitial lung disease, as well as in those undergoing immunomodifier drugs, i.e. glucocorticoids, mycophenolate-mofetil (MMF), and/or RTX. Therefore, we identified three main subgroups of ASD patients: (1) responders; (2) sub-optimal responders, and (3) non-responders. The latter two subgroups should be prioritized for the administration of a booster dose of vaccine or a different type of vaccine [15]. Here we report results concerning the vaccine immunogenicity in ASD patients after completion of the vaccination cycle including the booster dose.
2. Patients and methods
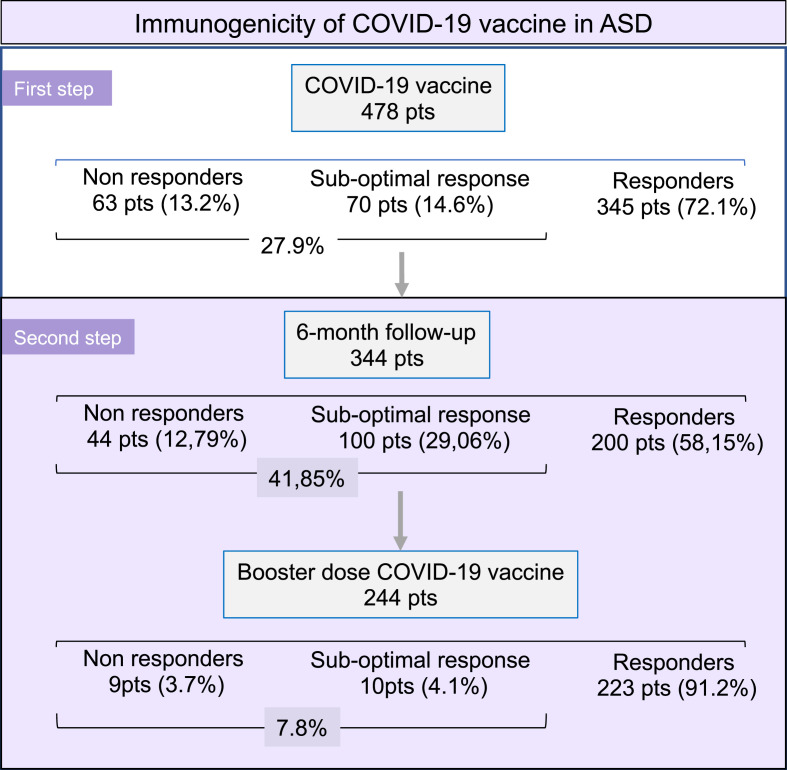
Our prospective, observational study evaluated the immunogenicity and safety of COVID-19 vaccines, as well as the possible role of ongoing immune-modifier treatments in a series of well-defined ASD population, collecting data from 21 Italian referral centers. In a previous report, we analyzed the vaccine immunogenicity in 478 ASD patients during the first 2–4 weeks after the first cycle of vaccination [15]. Here, we examined 244 patients who received the vaccine booster by detecting serum NAb before (time 2) and after (time 3) the booster dose administered after at least 6 months from the first vaccination cycle (Fig. 1 ). This subgroup included responders (n = 175), non-responders (n = 33), or with suboptimal response to COVID-19 vaccine at time 1 (n = 36; Fig. 1).
Fig. 1.
Flow-chart reporting the number of patients investigated for COVID-19 immunogenicity at different time-points.
Figure 1 legend: At the beginning, a series of 478 ASD patients were analyzed as regards the humoral immunogenicity of COVID-19 vaccine [15]; over one quarter (27.9%) of individuals developed valuable levels of serum NAb within the first month after the vaccination cycle (first step). During the present study (second step), we evaluated the NAb titers in 344 ASD patients after the first 6-month follow-up period; a marked increase of the absent/suboptimal responder rate (from 27.9% to 41.85%) was recorded. Successively, the booster dose of vaccine was administered to 244 ASD patients leading to a significant reduction of the impaired seroconversion rate.
The disease composition of the 244 ASD patients (M: 16.8%, 62 ± 14 years) was comparable to that of the initial ASD series (Table 1 ) and included: 48 seropositive rheumatoid arthritis (RA), 16 systemic lupus erythematosus (SLE), 155 systemic sclerosis (SSc), 21 cryoglobulinemic vasculitis (CV), and 4 patients with other forms of systemic vasculitis. In all cases, disease definition and clinical assessment were performed according to the updated classification criteria and current methodologies [16].
Table 1.
Serum anti-SARS-CoV-2 IgG neutralizing antibodies (NAb) in Autoimmune Systemic Diseases (ASD).at different time points.
| Pts no. | After vaccination (A) |
After 6 months (B) |
After booster (C) |
P | |
|---|---|---|---|---|---|
| Diseases | NAb titer BAU/mL, mean (SEM) | NAb titer BAU/mL, mean (SEM) | NAb titer BAU/mL, mean (SEM) | ||
| RA | 48 | 334,8 (±98,92) | 194,0 (±61,04) | 1444 (±136,9) | A vs B ns B vs C < 0.0001 A vs C < 0.0001 |
| SLE | 16 | 570,3 (±172,8) | 146,2 (±70,94) | 1785 (±255,7) | A vs B < 0.05 B vs C < 0.0001 A vs C < 0.0001 |
| SSc | 153 | 812,7 (±65,11) | 479,8 (±59,95) | 1528 (±99,28) | A vs B < 0.0001 B vs C < 0.0001 A vs C < 0.0001 |
| CV | 21 | 783,8 (±236,8) | 97,54 (±29,21) | 1442 (±241) | A vs B < 0.05 B vs C < 0.05 A vs C < 0.0001 |
| Other Vasculitis | 4 | 193,6 (±65,93) | 676,6 (±607,9) | 1349 (±406,3) | A vs B ns B vs C ns A vs C ns |
| ASD total∗ | 244 | 696,8 (±52,68) | 370,8 (±41,92) | 1527 (±74,16) | A vs B < 0.0001 B vs C < 0.0001 A vs C < 0.0001 |
| Controls | 502 | 1138 (±46,93) | 643.9 (±26.84) | 2470 (±10.74) | A vs B < 0.0001 B vs C < 0.0001 A vs C < 0.0001 |
| Compared to Controls: | Compared to Controls: | Compared to Controls: | |||
| ASD (p. <0.0001) | ASD (p. <0.0001) | ASD (p. <0.0001) | |||
| RA (p. <0.0001); SLE (p. 0,0023) | RA (p. <0,0001); SLE (p. <0,0001) | RA (p. <0,0001); SLE (p. <0,0001) | |||
| SSc (p. <0,0001); CV (p. 0,0028) | SSc (p. <0,0001); CV (p. <0,0001) | SSc (p. <0,0001); CV (p. <0,0001) |
RA: rheumatoid arthritis, SLE: systemic lupus erythematosus, SSc: systemic sclerosis, CV: cryoglobulinemic vasculitis.
A, B, and C: time 1, 2, and 3, respectively (see text).
*The group of 244 ASD patients who received either the first cycle and the booster dose of COVID-19 vaccine.
The study protocol included the collection of patients' data at the peri-vaccination as previously described [15]; in particular, it includes the patients’ clinical/epidemiological and therapeutic features, types of vaccine administered, adverse events and/or disease flares triggered by the vaccine injection. In addition, the peri-vaccination administration of immune-modifier medications followed the recommendations of the Italian Society of Rheumatology (https://www.reumatologia.it/vaccinazioni), considering the disease activity and major comorbidities of the single patient. Individuals with a history of allergic reactions to vaccinations, recent SARS-CoV-2 infection, and/or pregnancy were excluded by the study.
The immunogenicity of COVID-19 vaccines was evaluated by measuring the titer of NAb against SARS-CoV-2 trimeric spike S1/S2 glycoproteins on serum samples obtained within 2–4 weeks and 6 months after completion of the first vaccination cycle, and after the booster vaccine dose. The NAb levels were measured by means of SARS-CoV-2 IgG II Quant antibody test kit (Abbott Laboratories, Chicago, IL). As recommended by the World Health Organization (WHO), antibody titers are expressed as Binding Antibody Units (BAU)/ml, with a cut-off for positive testing of 7 BAU/ml. A serum titer of NAb 10× normal upper limit (≤70 BAU/ml) was classified as suboptimal response.
The study was performed in accordance with the principles of the Declaration of Helsinki, and it was approved by the Ethic Committee “Area Vasta Centro”, Florence, Italy (RETRO-CoV2 study code #17886_bio); all patients gave their informed consent before participation.
A series of 502 subjects (M: 27%, mean age 59 ± 14SD years) from the general population was used as the control group. As an additional exclusion criterion, individuals with a past diagnosis of any ASD and/or a past exposure for any reason to immunosuppressive therapies were also excluded from the control group.
COVID-19 vaccine was administered to both patients and controls by intramuscular injection in the deltoid muscle according to the manufacturer indications and Italian national guidelines; the BNT162b2 and mRNA-1273 vaccines were administered in 93.7% and 6.3% ASD patients, respectively, as well as in comparable percentages of control individuals.
2.1. Statistical analysis
Data are expressed as mean ± standard error of mean (SEM), or number (percentage) as appropriate. The Student's T test (Mann-Whitney for paired samples or Wilcoxon test for unpaired samples) was used for comparing means of continuous variables between two groups. Fisher's exact test was used to compare categorical variables between two groups. All tests were two tailed and a p value < 0.05 was considered significant. Analyses were performed using the GraphPad Software v 9.
3. Results
Table 1 reports the time course of serum NAb levels detected within 2–4 weeks after the first vaccination cycle (time 1), after six-month interval (time 2), and within 2–4 weeks following the booster dose administration (time 3). Significantly lower levels of NAb were observed in the whole patients’ series and in the single ASD subgroups at both time 1 and time 2, when compared to the control group (Table 1). The booster dose of vaccine produced a significant increase of NAb levels even compared to the first measurements; however, the difference with the control group remained statistically significant (patients 1527 ± 74.16 SEM BAU/mL vs controls 2470 ± 10.74SEM BAU/mL; p < 0.0001). Moreover, the cumulative percentage of ASD patients with absent or suboptimal response raised from 28.27% (69/244) of time 1–46.31% (113/244); p < 0.0001, OR 2.18 (CI: 1.49–3.18)) of time 2; while, the booster dose decreased the percentage of patients with deficient seroconversion (from 46.31% to 7.8%, p < 0.0001 OR 0.09 (CI: 0.05–0.16). However, the percentage of ASD patients with absent/suboptimal response to booster dose remained significantly higher when compared with the control subjects (19/244, 7.8% vs 1/502, 0.2%; p < 0.0001 OR 0.02 (CI: 0.003–0.13).
Demographic, clinical and treatment information on the 19 ASD patients with no response (n = 9) or suboptimal response (n = 10) to booster dose of COVID-19 vaccine is summarized in Table 2 ; over half of these individuals were affected by SSc (n = 9), followed by RA (n = 5), CV (n = 4), and SLE (n = 1).
Table 2.
ASD patients with absent or suboptimal seroconversion after booster administration of COVID-19 vaccine.
| ASD Patients |
NAb anti-COVID-19 |
||||||
|---|---|---|---|---|---|---|---|
| Sex/ | serum titer after | ||||||
| no. | Age | Diagnosis | Main Symptoms° | Treatments* | COVID-19 vaccine | 6 months | Booster dose |
| Non response | |||||||
| 1 | F/57 | SSc | dcSSc, SU | MMF, low dose CS | nr | nr | nr |
| 2 | F/57 | SSc | dcSSc, arthritis | MTX, ABA, low dose CS | nr | nr | nr |
| 3 | F/52 | SSc | dcSSc, overlapping SLE | RTX, low dose CS | nr | nr | nr |
| 4 | F/43 | SSc | dcSSc, ILD, SU | MMF, RTX, low dose CS | nr | nr | nr |
| 5 | F/52 | SSc | lcSSc, overlapping SLE | MMF | nr | nr | nr |
| 6 | M/83 | CV | SU, purpura, peripheral neuropathy | high dose CS | nr | nr | nr |
| 7 | F/78 | CV | SU, purpura, peripheral neuropathy, hyperviscosity s. | RTX | nr | nr | nr |
| 8 | F/72 | CV | purpura, B-NHL | Chemiotherapy | nr | nr | nr |
| 9 | M/75 | RA | polyarthritis | MTX, low dose CS | nr | nr | nr |
| Suboptimal response | |||||||
| 10 | M/60 | RA | polyarthritis | MTX | sub | sub | sub |
| 11 | F/45 | RA | overlapping SLE | RTX, HCQ, low dose CS | nr | nr | sub |
| 12 | M/73 | RA | polyarthritis, ILD | HCQ, low dose CS | sub | 286.5 | sub |
| 13 | F/74 | RA | polyarthritis, ILD | JAK, low dose CS | nr | nr | sub |
| 14 | F/22 | SLE | polyarthritis, thyroiditis | BEL, CyA, HCQ, low dose CS | 115.4 | nr | sub |
| 15 | F/86 | SSc | lcSSc, ILD, K | low dose CS | sub | nr | sub |
| 16 | F/71 | SSc | lcSSc | HCQ | 140.2 | sub | sub |
| 17 | F/56 | SSc | dcSSc, ILD | MMF | 768 | sub | sub |
| 18 | F/54 | SSc | lcSSc | MMF, RTX** | 675,7 | nr | sub |
| 19 | M/75 | CV | purpura, SU | RTX, low dose CS | nr | nr | sub |
ASD: autoimmune systemic diseases; COVID-19: coronavirus disease 2019; RA: rheumatoid arthritis; SSc: systemic sclerosis; SLE: systemic lupus erythematosus; CV: cryoglobulinemic vasculitis; lcSSc: limited cutaneous SSc; dcSSc: diffuse cutaneous SSc; SU: skin ulcers; ILD: interstitial lung disease; NAb: neutralizing antibodies; titre of NAb: BAU/mL; nr: non response (<7 BAU/mL); sub: suboptimal response (<70 BAU/mL) to COVID-19 vaccine. *treatments within the last 6 months and during the vaccination cycle: MTX: methotrexate; RTX: rituximab; MMF: mycophenolate mofetyl; ABA: abatacept; JAK: Janus kinase inhibitors; BEL: belimumab; CyA: cyclosporin A; TNF: anti-TNF-alpha inhibitors; HCQ: hydroxychloroquine; CS: corticosteroids; **RTX cycle administered after the first 2 doses of vaccine; nr: non-responder; sub: sub-optimal responder.
Moreover, all the non-responders (9/9) to booster COVID-19 vaccine were treated with immunomodifier drugs, including high dose corticosteroids, as well as 7/10 (70%) of those with sub-optimal response (Table 2).
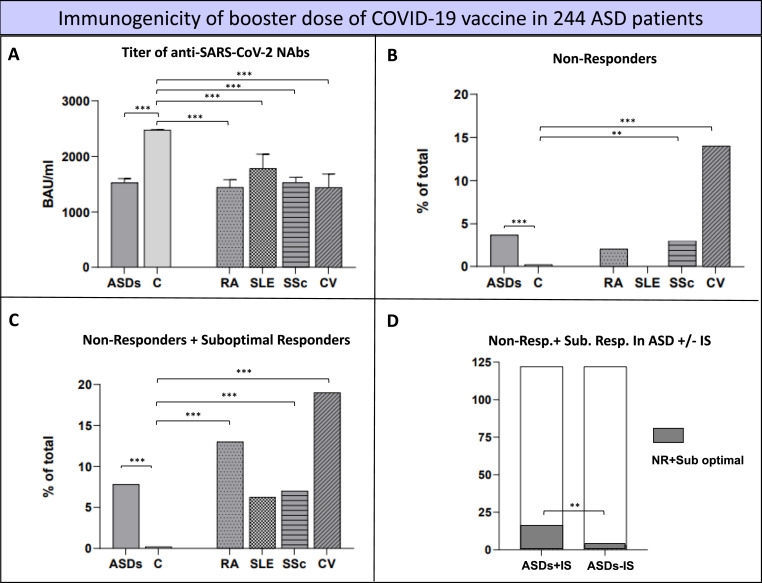
Fig. 2 summarizes the main clinical features of the 244 ASD patients after the booster dose of COVID-19 vaccine in the whole series and in each disease subgroup: Serum NAb titers (Fig. 2, panel A), percentages of no response (Fig. 2, panels B), percentages of no response or suboptimal response (Fig. 2, panels C), and the relationship between the seroconversion and the ongoing immunomodulating treatments (Fig. 2, panel D). In this respect, the cumulative number of patients with absent/suboptimal response was significantly higher in subjects undergoing immune-modifiers (16/122, 13.1% vs 3/122, 2.46% p = 0.0031; Fig. 2, panel D).
Fig. 2.
Booster dose immunogenicity in the 244 ASD patients compared to healthy controls.
Figure 2 legend: The figure focuses on the immunogenicity elicited by the booster dose of COVID-19 vaccine the main clinical features of the 244 ASD patients after the booster dose of COVID-19 vaccine. The serum levels of NAb are shown in the panel A, while panels B & C report the non-responder/suboptimal responder rates in the whole series and in each disease subgroups. Finally, the relationship of vaccine-related immunogenicity is correlated with the immunomodulating treatments in panel D. ASD patients showed significantly lower NAb titers compared to control group (ASD = 1527(±74.16) BAU/ml vs Controls = 2470(±10.74) BAU/ml p < 0.0001, panel A), as well the single ASD subgroups (RA = 1444(±136.9) BAU/ml, SLE = 1785(±255.7) BAU/ml, SSc = 1528(±99.28) BAU/ml, CV = 1442(±241) BAU/ml; always <0.0001). Similar findings was observed for the percentage of both non-responders (panel B) and sub-optimal responders (panel C). The cumulative number of patients with absent/suboptimal response was significantly higher in subjects treated with immune-modifiers compared to those without (panel D) (16/122(13.1%) and 3/122(2.46%) p = 0.0031). RA: rheumatoid arthritis, SLE: systemic lupus erythematosus, SSc: systemic sclerosis, CV: cryoglobulinemic vasculitis; IS: immuno-suppressors.
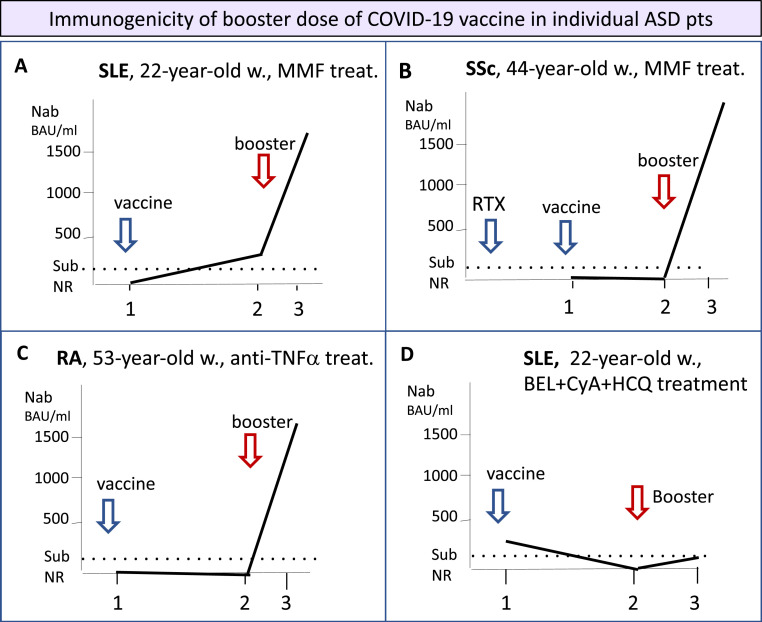
The trend of serum NAb titer during the whole vaccination cycle was quite unpredictable in ASD patients. Fig. 3 describes some representative examples: Panel A: a 22 year-old woman affected by severe SLE-glomerulonephritis on MMF therapy showed delayed seroconversion especially after the booster dose. Panel B: a 44 year-old woman affected by diffuse cutaneous SSc complicated by interstitial lung involvement undergoing long-term MMF (2.000 mg/day). Serum NAb were absent after the first 2 determinations (within the first 4 weeks after the initial vaccination and 6 months later), while strong seroconversion occurred after the booster dose of vaccine (BNT162b2). This late response might be correlated with a previous RTX treatment administered before the first vaccination cycle. Panel C: a 53 year-old woman following long-lasting treatment with anti-TNFα monoclonal antibody developed high NAb levels only after the administration of a booster dose of vaccine. Panel D: a 22-year-old woman with SLE treated with belimumab, hydroxychloroquine, cyclosporin A, and low-dose corticosteroids developed low NAb levels after the first vaccination cycle, followed by a complete absence of seroconversion after the booster dose of vaccine.
Fig. 3.
Immunogenicity of booster dose of COVID-19 vaccine in individual ASD patients.
Figure 3 legend: The immunogenicity to COVID-19 vaccines is largely variable and often unpredictable among ASD patients. It may be influenced by the single patient's conditions, namely the genetically-driven immune-system reactivity, older age, type of ASD, presence of comorbidities, and mostly by recent/ongoing immunomodifier treatments. The figure shows the response to first COVID-19 vaccination and to booster dose observed in different types of ASD. Panel A: a 22-year-old woman affected by systemic lupus erythematosus (SLE) with complicating severe glomerulonephritis treated since 2019 with mycophenolate mofetil (2 g/day) who developed a mild, delayed response after the first 2 doses of COVID-19 vaccine (BNT162b2), followed by a robust NAb production with the administration of a booster dose of the same vaccine. Panel B: a 44-year-old woman affected by diffuse cutaneous systemic sclerosis (SSc) complicated by interstitial lung involvement, undergoing long-term mycophenolate mofetil (2 g/day). The patients revealed as non-responder at the first 2 determination of serum NAb (within the first 4 weeks after the initial vaccination cycle and 6 months later), while a clear-cut seroconversion was recorded after the booster dose of vaccine (BNT162b2). This late response might be correlated with the previous cycle of rituximab treatment (10 months before the first vaccination). Panel C: a 53-year-old woman affected by rheumatoid arthritis (RA) undergoing long-term anti-TNFα treatment (Adalimumab). As observed in the patient described in panel B, the booster dose of COVID-19 vaccine (BNT162b2) was able to induce a valid seroconversion. Panel D: a 22-year-old woman affected by systemic lupus erythematosus (SLE) was undergoing long-term combined therapy with Belimumab, cyclosporin A, and hydroxychloroquine; the follow-up of serum NAb titers revealed a persistent inadequate response to COVID-19 vaccine (BNT162b2), including the booster dose administration. Timing 1: after the first 4 weeks from initial vaccination cycle; 2: after six-month follow-up; 3: within the first 4 weeks after booster dose.
The percentage of ASD patients reporting one or more side effects within four weeks after booster administration was 40% (97/244); the side effects were usually mild and transitory. Remarkably, ASD reactivation following the booster shot was observed in only 2% (5/244) of cases although the events were invariably self-limiting.
4. Discussion
The present study analyzed prospectively the immunogenicity effects of the entire anti-SARS-CoV-2 vaccination cycle in a series of frail patients with well-defined ASDs. The seroconversion induced by multiple doses of COVID-19 vaccine was evaluated after the first vaccination, after 6 months, and after the booster dose administration. Following the initial demonstration of impaired immunogenicity induced by the first vaccination cycle [15], the present study highlights a dramatic drop in serum NAb levels at 6 months along with the positive peak triggered by the booster dose of vaccine. Nevertheless, NAb titers remained on average lower compared to the general population even after the booster vaccine, while a relevant proportion of patients showed absent or suboptimal response. In many patients this deficiency could be, at least in part, attributable to the ongoing immune-modifier treatments, which have been correlated with an impaired immunogenicity towards vaccination [[10], [11], [12], [13],[17], [18], [19]].
Few studies regarding the effects of the third dose in ASD subjects are available in the literature [[20], [21], [22], [23], [24], [25], [26]]. In addition, the comparison between our results and previous reports is quite difficult because of several differences regarding the size and disease composition of the patients’ series, the timing of the NAb dosages, and the concomitant treatments [[20], [21], [22], [23], [24], [25], [26]].
The widest analysis so far performed is an Israeli national cohort study on 127,928 ASD patients reporting that people receiving mRNA COVID-19 vaccine and, particularly, the booster dose, had a better COVID-19 outcome. This finding might indirectly suggest an effective vaccine-driven immune protection [27].
A number of reports, generally on small patients series, often focusing on single autoimmune disorders, underlined effective seroconversion produced by the booster dose of vaccine [20,21,[24], [25], [26]], as well as the weak response to the booster vaccine in ASD patients previously treated with RTX or MMF [21,22,[24], [25], [26]].
Of note, the percentage of non-responders or suboptimal-responders ASD patients who experienced an increase of serum NAb titers after the booster dose is extremely variable in the previous reports (from 16.3% to 92%) [[20], [21], [22], [23], [24], [25], [26]]. Thus, it is possible to hypothesize that the reported discrepancies in the improvement of booster-related immunogenicity may reflect a variable contribution of several factors; in particular, the differences among various patients’ series investigated in ASD subtype composition and/or in the treatments employed in the peri-vaccination period.
In our long-term observational study, absent/suboptimal production of NAb observed in over 1/4 ASD patients was already recorded soon after the first vaccine cycle. Such weak response further worsened at the end of the first 6-month period, followed by a robust recovery of immunogenicity after the booster dose of vaccine. The residual percentage (7.8%) of ASD patients with persistent absent/suboptimal production of NAb remains significantly higher than that observed in controls (0.2%). Therefore, ASD represents a harmful condition in the clinical practice, especially if neglected in individual patients.
The immune system derangement underlying ASD might explain only in part the weak/absent response to the booster vaccine; an increasing number of clinical investigations including the present observations pointed out the role of ongoing immune-modulating treatments on both T- and B-cell response to vaccine. In this respect, a wide panel of conventional (methotrexate, leflunomide, azathioprine, sulfasalazine, hydroxychloroquine, and high-dose corticosteroids) and biological disease-modifying anti-rheumatic drugs (inhibitors of TNFα, IL1, IL6, Janus-kinases, T- and B lymphocytes such as abatacept, rituximab, and belimumab) may more or less markedly weaken the immune response to vaccine antigens. An individualized therapeutic peri-vaccination strategy for ASD patients should improve the overall vaccine immunogenicity, by, for example, tapering/discontinuing or changing the immunomodulating drug. The role of RTX in blunting the humoral response to vaccines for long-lasting period is documented, even when administered beyond the 6-month interval prior the vaccination [5,17,18,28]. The putative role of low-dose glucocorticoids [11,13], methotrexate [[11], [12], [13],29,30], and abatacept [13] on COVID-19 vaccine immunogenicity remains still controversial. Other drugs, such as belimumab or cyclosporin A, seem to do not interfere with antibody responses [30], even if our observation of the 22-year-old women affected by SLE might suggest a contribution of these drugs in the impaired seroconversion also after the booster vaccine.
Therefore, the awareness of both disease-related and iatrogenic risk factors may drive the patients’ management, though the inadequate response to booster vaccine remains quite unpredictable in the single patient as suggested by our detailed analysis of some patients with inadequate seroconversion during the vaccination cycle. As a consequence, it may be crucial to early identify individuals with absent/suboptimal seroconversion who are at high risk to be infected and to develop severe COVID-19. The latter event is more frequently noticed in older ASD patients with disease-related complications such as interstitial lung involvement [6,8] and/or with major comorbidities [31].
The management of ASD patients during the ongoing pandemic should include a careful monitoring of serum NAb levels since the first dose of vaccine administration. Some preliminary observations suggested that the blunted humoral immune response to vaccines, mostly caused by iatrogenic factors, may be counterbalanced by a preserved T-cell response [32]. The introduction in the routine practice of recently developed procedures evaluating the T-cell response [33] may usefully integrate the serum NAb detection in the assessment of the whole immunogenicity elicited by COVID-19 vaccines.
5. Conclusion
Based on present and other recent reports on the effect of the vaccine booster dose we may suggest the following provisional measures:
-
•
serum levels of NAb should be evaluated in all ASD patients within the first 4 weeks after both the first vaccination and the booster dose(s); the patients might be classified as full responders, suboptimal responders, or non-responders;
-
•
T-cell response to COVID-19 vaccine should be evaluated in patients with absent/suboptimal NAb production persisting after the booster dose;
-
•
in patients with deficient response to COVID-19 vaccine a tight control of the ASD is highly advisable; it should include the monitoring of disease activity and the adjustment of treatment such as tapering/discontinuation or delayed therapeutic sessions;
-
•
finally, in individual patients without seroconversion after booster vaccine, especially in the presence of risk factors for severe COVID-19 (older age, active disease, disease-related interstitial lung involvement, and/or major comorbidities), the administration of a different type of booster vaccine, as well as preemptive treatments with monoclonal anti-SARS-CoV-2 antibodies and/or novel antiviral drugs should be carefully considered [[34], [35], [36], [37]].
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Authors’ statements
Clodoveo Ferri: conceptualisation, study design, supervision, writing, investigation, methodology, project administration, literature search; Laura Gragnani: writing, methodology, investigation, literature search; Vincenzo Raimondo: data collection; Marcellla Visentini: data collection; Dilia Giuggioli: data collection; Serena Lorini: formal analysis, figures; Rosario Foti: data collection; Fabio Cacciapaglia: data collection; Maurizio Caminiti: data collection; Domenico Olivo: data collection; Giovanna Cuomo: data collection; Roberta Pellegrini: data collection; Erika Pigatto: data collection; Teresa Urraro: data collection; Caterina Naclerio: data collection; Antonio Tavoni: data collection; Lorenzo Puccetti: data collection; Ilaria Cavazzana: data collection; Piero Ruscitti: data collection; Marta Vadacca: data collection; Francesca La Gualana: data collection; Franco Cozzi: data collection; Amelia Spinella: data collection; Elisa Visalli: data collection; Ylenia Dal Bosco: data collection; Giorgio Amato: data collection; Francesco Masini: data collection; Giuseppa Pagano Mariano: data collection; Raffaele Brittelli: data collection; Vincenzo Aiello: data collection; Daniela Scorpiniti: data collection; Giovanni Rechichi: data collection; Giuseppe Varcasia: data collection; Monica Monti: data collection; Giusy Elia: data collection; Franco Franceschini: data collection; Milvia Casato: data collection; Francesco Ursini: data collection; Roberto Giacomelli: data collection; Poupak Fallahi: data collection; Stefano Angelo Santini: methodology; Florenzo Iannone data collection; Carlo Salvarani: data collection; Anna Linda Zignego: data collection; Alessandro Antonelli: conceptualisation, writing. All authors critically revised the paper and approved the submitted version.
Data availability
Data will be made available on request.
References
- 1.Pablos J.L., Galindo M., Carmona L., Lledó A., Retuerto M., Blanco R., et al. Clinical outcomes of hospitalised patients with COVID-19 and chronic inflammatory and autoimmune rheumatic diseases: a multicentric matched cohort study. Ann. Rheum. Dis. 2020;79:1544–1549. doi: 10.1136/annrheumdis-2020-218296. [DOI] [PubMed] [Google Scholar]
- 2.D'Silva K.M., Jorge A., Cohen A., McCormick N., Zhang Y., Wallace Z.S., et al. COVID-19 outcomes in patients with systemic autoimmune rheumatic diseases compared to the general population: a US multicenter, comparative cohort study. Arthritis Rheumatol. 2021;73:914–920. doi: 10.1002/art.41619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ferri C., Giuggioli D., Raimondo V., L'Andolina M., Tavoni A., Cecchetti R., et al. COVID-19 and rheumatic autoimmune systemic diseases: report of a large Italian patients series. Clin. Rheumatol. 2020;39:3195–3204. doi: 10.1007/s10067-020-05334-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Antonelli A., Fallahi P., Elia G., Ragusa F., Paparo S.R., Mazzi V., et al. Effect of the COVID-19 pandemic on patients with systemic rheumatic diseases. Lancet Rheumatol. 2021;3:e675–e676. doi: 10.1016/S2665-9913(21)00243-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fagni F., Simon D., Tascilar K., Schoenau V., Sticherling M., Neurath M.F., et al. COVID-19 and immune-mediated inflammatory diseases: effect of disease and treatment on COVID-19 outcomes and vaccine responses. Lancet Rheumatol. 2021;3:e724–e736. doi: 10.1016/S2665-9913(21)00247-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ferri C., Giuggioli D., Raimondo V., L’Andolina M., Dagna L., Tavoni A., et al. Covid-19 and rheumatic autoimmune systemic diseases: role of pre-existing lung involvement and ongoing treatments. Curr. Pharmaceut. Des. 2021:4245–4252. doi: 10.2174/1381612827666210903103935. [DOI] [PubMed] [Google Scholar]
- 7.Freites Nuñez D.D., Leon L., Mucientes A., Rodriguez-Rodriguez L., Font Urgelles J., Madrid García A., et al. Risk factors for hospital admissions related to COVID-19 in patients with autoimmune inflammatory rheumatic diseases. Ann. Rheum. Dis. 2020;79:1393–1399. doi: 10.1136/annrheumdis-2020-217984. [DOI] [PubMed] [Google Scholar]
- 8.Ferri C., Giuggioli D., Raimondo V., Dagna L., Riccieri V., Zanatta E., et al. COVID-19 and systemic sclerosis: clinicopathological implications from Italian nationwide survey study. Lancet Rheumatol. 2021;3:e166–e168. doi: 10.1016/S2665-9913(21)00007-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.D'Amelio R., Asero R., Cassatella M.A., Laganà B., Lunardi C., Migliorini P., et al. Anti-COVID-19 vaccination in patients with autoimmune-autoinflammatory disorders and primary/secondary immunodeficiencies: the position of the task force on behalf of the Italian immunological societies. Biomedicines. 2021;9 doi: 10.3390/biomedicines9091163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Geisen U.M., Berner D.K., Tran F., Sümbül M., Vullriede L., Ciripoi M., et al. Immunogenicity and safety of anti-SARS-CoV-2 mRNA vaccines in patients with chronic inflammatory conditions and immunosuppressive therapy in a monocentric cohort. Ann. Rheum. Dis. 2021;80:1306–1311. doi: 10.1136/annrheumdis-2021-220272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ruddy J.A., Connolly C.M., Boyarsky B.J., Werbel W.A., Christopher-Stine L., Garonzik-Wang J., et al. High antibody response to two-dose SARS-CoV-2 messenger RNA vaccination in patients with rheumatic and musculoskeletal diseases. Ann. Rheum. Dis. 2021;80:1351–1352. doi: 10.1136/annrheumdis-2021-220656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Braun-Moscovici Y., Kaplan M., Braun M., Markovits D., Giryes S., Toledano K., et al. Disease activity and humoral response in patients with inflammatory rheumatic diseases after two doses of the Pfizer mRNA vaccine against SARS-CoV-2. Ann. Rheum. Dis. 2021;80:1317–1321. doi: 10.1136/annrheumdis-2021-220503. [DOI] [PubMed] [Google Scholar]
- 13.Furer V., Eviatar T., Zisman D., Peleg H., Paran D., Levartovsky D., et al. Immunogenicity and safety of the BNT162b2 mRNA COVID-19 vaccine in adult patients with autoimmune inflammatory rheumatic diseases and in the general population: a multicentre study. Ann. Rheum. Dis. 2021;80:1330–1338. doi: 10.1136/annrheumdis-2021-220647. [DOI] [PubMed] [Google Scholar]
- 14.Boyarsky B.J., Ruddy J.A., Connolly C.M., Ou M.T., Werbel W.A., Garonzik-Wang J.M., et al. Antibody response to a single dose of SARS-CoV-2 mRNA vaccine in patients with rheumatic and musculoskeletal diseases. Ann. Rheum. Dis. 2021;80:1098–1099. doi: 10.1136/annrheumdis-2021-220289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ferri C., Ursini F., Gragnani L., Raimondo V., Giuggioli D., Foti R., et al. Impaired immunogenicity to COVID-19 vaccines in autoimmune systemic diseases. High prevalence of non-response in different patients' subgroups. J. Autoimmun. 2021;125 doi: 10.1016/j.jaut.2021.102744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bijlsma J.W., editor. EULAR Compendium on Rheumatic Diseases. New Edition ed. BMJ Publishing Group Ltd; London: 2018. [Google Scholar]
- 17.Bonelli M.M., Mrak D., Perkmann T., Haslacher H., Aletaha D. SARS-CoV-2 vaccination in rituximab-treated patients: evidence for impaired humoral but inducible cellular immune response. Ann. Rheum. Dis. 2021;80:1355–1356. doi: 10.1136/annrheumdis-2021-220408. [DOI] [PubMed] [Google Scholar]
- 18.Spiera R., Jinich S., Jannat-Khah D. Rituximab, but not other antirheumatic therapies, is associated with impaired serological response to SARS- CoV-2 vaccination in patients with rheumatic diseases. Ann. Rheum. Dis. 2021;80:1357–1359. doi: 10.1136/annrheumdis-2021-220604. [DOI] [PubMed] [Google Scholar]
- 19.Moor M.B., Suter-Riniker F., Horn M.P., Aeberli D., Amsler J., Möller B., et al. Humoral and cellular responses to mRNA vaccines against SARS-CoV-2 in patients with a history of CD20 B-cell-depleting therapy (RituxiVac): an investigator-initiated, single-centre, open-label study. Lancet Rheumatol. 2021:e789–e797. doi: 10.1016/S2665-9913(21)00251-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Assawasaksakul T., Sathitratanacheewin S., Vichaiwattana P., Wanlapakorn N., Poovorawan Y., Kittanamongkolchai W. vol. 7. RMD Open; 2021. (Immunogenicity, Safety and Reactogenicity of a Heterogeneous Booster Following the CoronaVac Inactivated SARS-CoV-2 Vaccine in Patients with SLE: a Case Series). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jyssum I., Kared H., Tran T.T., Tveter A.T., Provan S.A., Sexton J., et al. Humoral and cellular immune responses to two and three doses of SARS-CoV-2 vaccines in rituximab-treated patients with rheumatoid arthritis: a prospective, cohort study. Lancet Rheumatol. 2022;4:e177–e187. doi: 10.1016/S2665-9913(21)00394-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Speer C., Töllner M., Benning L., Klein K., Bartenschlager M., Nusshag C., et al. Third COVID-19 vaccine dose with BNT162b2 in patients with ANCA-associated vasculitis. Ann. Rheum. Dis. 2022:593–595. doi: 10.1136/annrheumdis-2021-221747. [DOI] [PubMed] [Google Scholar]
- 23.Connolly C.M., Teles M., Frey S., Boyarsky B.J., Alejo J.L., Werbel W.A., et al. Booster-dose SARS-CoV-2 vaccination in patients with autoimmune disease: a case series. Ann. Rheum. Dis. 2021:291–293. doi: 10.1136/annrheumdis-2021-221206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schmiedeberg K., Vuilleumier N., Pagano S., Albrich W.C., Ludewig B., Kempis J.V., et al. Efficacy and tolerability of a third dose of an mRNA anti-SARS-CoV-2 vaccine in patients with rheumatoid arthritis with absent or minimal serological response to two previous doses. Lancet Rheumatol. 2022;4:e11–e13. doi: 10.1016/S2665-9913(21)00328-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Syversen S.W., Jyssum I., Tveter A.T., Tran T.T., Sexton J., Provan S.A., et al. Arthritis Rheumatol; 2022. Immunogenicity and Safety of Standard and Third Dose SARS-CoV-2 Vaccination in Patients on Immunosuppressive Therapy. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Connolly C.M., Chiang T.P., Teles M., Frey S., Alejo J.L., Massie A., et al. Factors associated with poor antibody response to third-dose SARS-CoV-2 vaccination in patients with rheumatic and musculoskeletal diseases. Lancet Rheumatol. 2022:e382–e384. doi: 10.1016/S2665-9913(22)00065-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bieber A., Sagy I., Novack L., Brikman S., Abuhasira R., Ayalon S., et al. BNT162b2 mRNA COVID-19 vaccine and booster in patients with autoimmune rheumatic diseases: a national cohort study. Ann. Rheum. Dis. 2022:1028–1035. doi: 10.1136/annrheumdis-2021-221824. [DOI] [PubMed] [Google Scholar]
- 28.Simon D., Tascilar K., Schmidt K., Manger B., Weckwerth L., Sokolova M., et al. Arthritis Rheumatol; 2021. Brief Report: Humoral and Cellular Immune Responses to SARS-CoV-2 Infection and Vaccination in B Cell Depleted Autoimmune Patients. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Connolly C.M., Boyarsky B.J., Ruddy J.A., Werbel W.A., Christopher-Stine L., Garonzik-Wang J.M., et al. Absence of humoral response after two-dose SARS-CoV-2 messenger RNA vaccination in patients with rheumatic and musculoskeletal diseases: a case series. Ann. Intern. Med. 2021;174:1332–1334. doi: 10.7326/M21-1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ammitzbøll C., Bartels L.E., Bøgh Andersen J., Risbøl Vils S., Elbaek Mistegård C., Dahl Johannsen A., et al. Impaired antibody response to the BNT162b2 messenger RNA coronavirus disease 2019 vaccine in patients with systemic lupus erythematosus and rheumatoid arthritis. ACR Open Rheumatol. 2021;3:622–628. doi: 10.1002/acr2.11299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Eder L., Croxford R., Drucker A.M., Mendel A., Kuriya B., Touma Z., et al. COVID-19 hospitalizations, intensive care unit stays, ventilation, and death among patients with immune-mediated inflammatory diseases compared to controls. J. Rheumatol. 2022;49:523–530. doi: 10.3899/jrheum.211012. [DOI] [PubMed] [Google Scholar]
- 32.Bitoun S., Henry J., Desjardins D., Vauloup-Fellous C., Dib N., Belkhir R., et al. Arthritis Rheumatol; 2021. Rituximab Impairs B Cell Response but Not T Cell Response to COVID-19 Vaccine in Autoimmune Diseases. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Farroni C., Picchianti-Diamanti A., Aiello A., Nicastri E., Laganà B., Agrati C., et al. Kinetics of the B- and T-cell immune responses after 6 Months from SARS-CoV-2 mRNA vaccination in patients with rheumatoid arthritis. Front. Immunol. 2022;13 doi: 10.3389/fimmu.2022.846753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gandhi R.T., Malani P.N., Del Rio C. COVID-19 therapeutics for nonhospitalized patients. JAMA. 2022;327:617–618. doi: 10.1001/jama.2022.0335. [DOI] [PubMed] [Google Scholar]
- 35.Sendi P., Razonable R.R., Nelson S.B., Soriano A., Gandhi R.T. Clin Microbiol Infect; 2022. First-generation Oral Antivirals against SARS-CoV-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wen W., Chen C., Tang J., Wang C., Zhou M., Cheng Y., et al. Efficacy and safety of three new oral antiviral treatment (molnupiravir, fluvoxamine and Paxlovid) for COVID-19:a meta-analysis. Ann. Med. 2022;54:516–523. doi: 10.1080/07853890.2022.2034936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.NIH. Therapeutic Management of Nonhospitalized Adults with COVID-19.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.