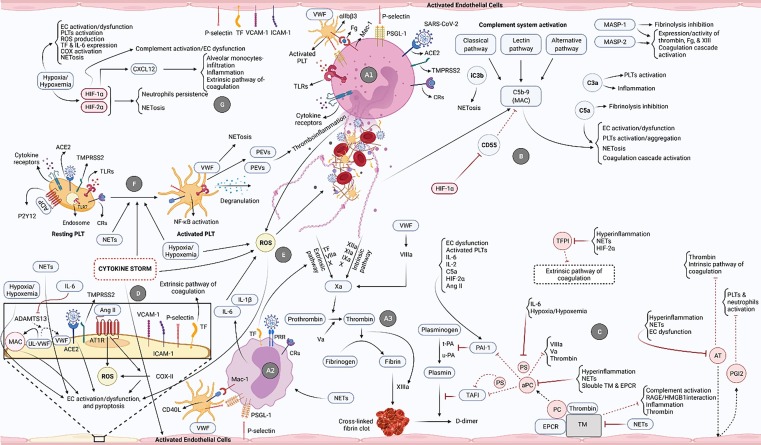
Fig. 1.
The most pivotal processes/pathways involved in the development of uncontrolled immunothrombosis in the critical COVID-19. A1) SARS-CoV-2/ACE2 interaction and ligation of different receptors of neutrophils provoke their activation and NETs formation. These cells then interact with PLTs and ECs and release NETs. A2) Ligation of diverse receptors of monocytes (e.g., pattern recognition receptors (PRRs) and CRs), their interplay with ECs and PLTs, and also released NETs cause the activation of monocytes followed by high-levels production of pro-inflammatory cytokines. A3) NETs are decorated with TF on the one hand and induce factor XII (FXII) on the other, respectively resulting in the activation of extrinsic and intrinsic coagulation pathways and formation of cross-linked fibrin clots. TF is also expressed on the monocytes and activates the extrinsic coagulation pathway. B) Activation of the complement system through all three pathways can lead to the formation of MAC, production of C3a, C5a, iC3b, etc. each of which directly and indirectly causes NETosis induction, inflammation development, and fibrinolysis inhibition. The MASP-1 and MASP-2 molecules also play key roles in the coagulation induction and fibrinolysis restraining. C) During the fibrinolysis process, plasminogen is converted into plasmin under the influence of t-PA and u-PA eventually leading to the breakdown of the cross-linked fibrin clots and generation of D-dimer. PAI-1 inhibits these plasminogen activators and is activated by several factors involved in uncontrolled immunothrombosis. Endogenous anticoagulants also play central roles provoking fibrinolysis and preventing the formation of the clots. In addition to the induction of some anti-inflammatory roles, TM forms a complex with thrombin and activates the PC (bound to the endothelial protein C receptor (EPCR)), whereas aPC inhibits the coagulation mediators in different ways. Another anticoagulant, PS, directly and indirectly (as an aPC's co-factor) participates in the fibrinolysis process. AT and TFPI also inhibit the coagulation pathways. In addition, AT restricts the NETosis through the induction of prostaglandin I2 (PGI2) production by the ECs. Involved in the uncontrolled immunothrombosis, multiple factors restrain the functions of anticoagulants resulting in inefficient fibrinolysis. D) SARS-CoV-2/ACE2 and Ang II/AT1R interactions, NETs, hypoxia/hypoxemia, and cytokine storm cause the activation/dysfunction and pyroptosis of ECs. The inhibition of ADAMTS13 by IL-6 also underlies the reduced conversion of ultra-large VWF (UL-VWF) into the normal size. This molecule then acts as a scaffold for the formation of MAC contributing to the ECs damage. The expression of TF and adhesion molecules by ECs is followed by the activation of the extrinsic coagulation pathway and NETosis. E) The high-levels production of ROS, induced by neutrophils, cytokine storm, hypoxia/hypoxemia, monocytes-released pro-inflammatory cytokines, etc., can provoke NETosis and ECs damage. F) SARS-CoV-2/ACE2 and endosomal TLR7/virus single-strand RNA (ssRNA) interactions, and also the ligation of different receptors of PLTs can cause their activation leading to the NETosis induction, PLT extracellular vesicles (PEVs) release, NF-κB pathway activation, and PLTs degranulation. The released NETs, cytokine storm, and hypoxia/hypoxemia are the other stimulants of PLTs activation. G) Hypoxia/hypoxemia stimulates different processes involved in the uncontrolled immunothrombosis and also mediates the production of HIF-1α and HIF-2α. These two factors perpetuate NETosis. HIF-1α also induces the activities of CXCL12 and the complement system. Therefore, it can proceed the ECs dysfunction leading to excessive NETosis, inflammation, and coagulation.