Abstract
Background
Clear cell sarcoma (CCS) is a translocated aggressive malignancy with a high incidence of metastases and poor prognosis. There are few studies describing the activity of systemic therapy in CCS. We report a multi-institutional retrospective study of the outcomes of patients with advanced CCS treated with systemic therapy within the World Sarcoma Network (WSN).
Materials and methods
Patients with molecularly confirmed locally advanced or metastatic CCS treated with systemic therapy from June 1985 to May 2021 were included. Baseline demographic and treatment information, including response by Response Evaluation Criteria in Solid Tumours (RECIST) 1.1, was retrospectively collected by local investigators. Descriptive statistics were carried out.
Results
Fifty-five patients from 10 institutions were included. At diagnosis, the median age was 30 (15-73) years and 24% (n = 13/55) had metastatic disease. The median age at diagnosis was 30 (15-73) years. Most primary tumours were at aponeurosis (n = 9/55, 16%) or non-aponeurosis limb sites (n = 17/55, 31%). The most common fusion was EWSR1–ATF1 (n = 24/55, 44%). The median number of systemic therapies was 1 (range 1-7). The best response rate was seen for patients treated with sunitinib (30%, n = 3/10), with a median progression-free survival of 4 [95% confidence interval (CI) 1-7] months. The median overall survival for patients with advanced/metastatic disease was 15 months (95% CI 3-27 months).
Conclusions
Soft tissue sarcoma-type systemic therapies have limited benefit in advanced CCS and response rate was poor. International, multicentre prospective translational studies are required to identify new treatments for this ultra-rare subtype, and access to early clinical trial enrolment remains key for patients with CCS.
Key words: clear cell sarcoma, CCS, EWSR1–ATF1, soft tissue sarcoma, systemic therapy, chemotherapy, sunitinib
Highlights
-
•
This is the largest reported series of advanced CCS patients treated with systemic therapy.
-
•
The activity of sarcoma-type systemic therapy is poor and modest responses were seen only with sunitinib.
-
•
Effective therapies are needed to improve outcomes for patients with this ultra-rare sarcoma type.
Introduction
Clear cell sarcoma (CCS) is an ultra-rare soft tissue sarcoma that often affects young adults with no sex preponderance.1, 2, 3 It often originates in limbs, specifically near aponeuroses and tendons.1 CCS is molecularly defined by reciprocal translocations involving EWSR1, with EWSR1–ATF1 [t(12;22)(q13;q12)] significantly more commonly seen than EWSR1–CREB1 [t(2;22)(q34;q12)].2,4
Driven by EWSR1–ATF1, microphthalmia-associated transcription factor (MITF) has been shown to be amplified in CCS.5 MITF plays a key role in regulating genes involved in cell growth, including BCL2 and MET.5,6 Despite this interesting biological rationale, treatment of 11 patients with CCS with the MET inhibitor tivantinib demonstrated an overall response rate (ORR) of only 9% (n = 1/11) and a disease control rate (DCR) of 36% (n = 4/11)5 and a median progression-free survival (mPFS) of 1.9 months.5 Another phase II trial with the MET inhibitor crizotinib (CREATE) showed an ORR of 3.8% (n = 1/28) and a DCR of 67.9% (n = 19/28), with an mPFS of 4.3 months.7 Tissue microarrays from patients enrolled in the CREATE trial8 demonstrated high levels of activation of pathways downstream of MET, including Grb2-associated binding protein 1 and mitogen-activated protein kinase, and copy number alterations in the region of MYC and CDKN2A.8
Whilst the pathophysiology of CCS oncogenesis is actively investigated, there is a limited real-world understanding of the impact of systemic therapies on patients with advanced CCS as it is not well described in the literature. Small retrospective studies have reported that conventional chemotherapy used for soft tissue sarcoma has limited activity.9 We aimed to review the outcomes of patients with CCS treated with systemic therapies within the World Sarcoma Network (WSN) to further understand treatment for this ultra-rare sarcoma type.
Materials and methods
Institutional ethics approval (RMH SE1020) was mandated, and data transfer agreements were obtained between the Royal Marsden Hospital and participating institutions. Adults (age >15 years) treated with systemic therapy for advanced (unresectable or metastatic) CCS were identified from June 1985 to May 2021. Patients were identified from 10 participating sarcoma reference centres across 9 countries within the WSN. Written informed consent to treatment was obtained as per local standard of care.
Molecular confirmation of the diagnosis (EWSR1–ATF1, EWSR1–CREB1, or EWSR1 fusion with an unknown partner) was mandatory for patient inclusion. Demographics and treatment information were retrospectively collected by investigators at each site. Radiological treatment response was retrospectively assessed through imaging review, using the same cut-off values defined by Response Evaluation Criteria in Solid Tumours Version 1.1 (RECIST 1.1). Imaging was carried out as per institutional standards, which is generally every 2-3 cycles of therapy.
Descriptive statistics were applied, and mPFS and median overall survival (mOS) were calculated using the Kaplan–Meier method using SPSS statistics version 27 (IBM, Armonk, NY). Progression-free survival was defined as the time from the start of systemic therapy to radiological progression defined by RECIST 1.1. If radiological progression was unable to be obtained but there was overt clinical progression, then the date of clinical progression was used. Patients who remained on treatment and had not progressed at the time of data cut-off were deemed non-assessable. Overall survival was defined as the time from the diagnosis of metastatic disease to death from any cause. Patients who were alive at the data cut-off or were lost to follow-up were censored.
Results
Fifty-five patients were identified from 10 institutions (Table 1) with a median follow-up of 22 months [95% confidence interval (CI) 16-24 months]. Molecular fusions included EWSR1–ATF1 (n = 24), EWSR1–CREB1 (n = 11), and EWSR1 fusion with an unknown partner (n = 24). The median age at diagnosis was 30 (range 15-73) years, there was an equal gender distribution, and most patients were of white ethnicity (47%). Over half of the patients had primary tumours of limbs. One-quarter (n = 13) of patients had metastatic disease at diagnosis. The median time to development of metastatic disease for those who presented with localized disease at diagnosis (n = 42) was 15 months (range 0-171 months).
Table 1.
Baseline characteristics of patients treated with systemic therapy
| n = 55 (%) | |
|---|---|
| Median age (range), years | 30 (15-73) |
| Gender | |
| Male | 28 (51) |
| Female | 27 (49) |
| Ethnicity | |
| White | 26 (47) |
| Black | 2 (4) |
| Hispanic | 7 (13) |
| Asian | 5 (9) |
| Unknown | 15 (27) |
| Primary tumour site | |
| Aponeurosis | 13 (24) |
| Non-aponeurosis limb | 18 (33) |
| Head and neck | 1 (2) |
| Intra-abdominal | 7 (13) |
| Thorax | 4 (7) |
| Pelvis | 3 (6) |
| Other | 9 (13) |
| Fusion status | |
| EWSR1-ATF1 | 24 (44) |
| EWSR1–CREB1 | 11 (20) |
| EWSR1–unknown partner | 20 (36) |
| Stage at diagnosis | |
| Localized | 42 (76) |
| Metastatic/unresectable | 13 (24) |
| Time to development of metastatic disease (range), months | |
| n = 42 | 15 (0-171) |
| Site of metastasis | |
| Lung | 26 (55) |
| Bone | 20 (36) |
| Liver | 6 (11) |
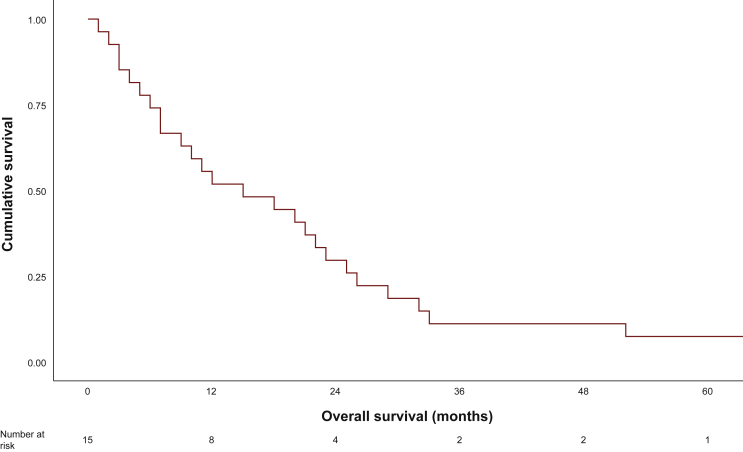
Patients were treated with a variety of systemic therapies (Table 2). The most commonly used included conventional sarcoma chemotherapy regimens (∼60% of patients with doxorubicin-based chemotherapy). The median number of systemic treatments used was 1 (range 1-7). The highest response rate was seen with sunitinib (n = 3/10, 30%), gemcitabine-based (n = 2/13, 15%), and doxorubicin-based (n = 4/34, 12%) chemotherapy. The mPFS for the entire cohort was 2 months (95% CI 1.2-2.7 months). The mOS for patients with advanced disease was 15 months (range 1-27 months) (Figure 1).
Table 2.
Response to systemic therapy
| Number treated (n = 55) | Best response by RECIST 1.1, n (%) |
Median PFS (95% CI), months | |||||
|---|---|---|---|---|---|---|---|
| Complete response | Partial response | Stable disease | Progressive disease | Not evaluable | |||
| Doxorubicin based | 34 | 0 | 4 (12) | 15 (44) | 12 (22) | 3 (9) | 3 (0.9-4) |
| Pazopanib | 16 | 0 | 0 | 3 (19) | 9 (56) | 4 (25) | 1 (0-2) |
| Gemcitabine based | 13 | 0 | 2 (15%) | 4 (31) | 6 (46) | 1 (8) | 3 (1-5) |
| Sunitinib | 10 | 0 | 3 (30) | 2 (20) | 4 (40) | 1 (10) | 4 (1-7) |
| Dacarbazine | 9 | 0 | 0 | 2 (22) | 5 (56) | 2 (22) | 2 (NA) |
| Trabectedin | 7 | 0 | 0 | 1 (14) | 6 (86) | 0 | 1 (0.3-4) |
| Crizotinib | 5 | 0 | 0 | 1 (20) | 4 (80) | 0 | 2 (0.9-3) |
| Checkpoint inhibitor | 5 | 0 | 0 | 2 (40) | 3 (60) | 0 | 2 (0-6) |
| Ifosfamide | 5 | 0 | 0 | 0 | 4 (80) | 1 (20) | 1 (NA) |
CI, confidence interval; NA, not applicable; PFS, progression-free survival.
Figure 1.
Median overall survival of metastatic/advanced patients.
Discussion
CCS is an ultra-rare sarcoma subtype.10 To our knowledge, this is the largest reported molecularly defined cohort of patients with advanced CCS treated with systemic therapy. The median age of our cohort was younger and patient gender more balanced than seen in the CREATE trial,7 but our cohort was otherwise similar to other published large retrospective cohorts.11,12 Most patients had aponeurosis or limb primary tumour site, in line with the available literature.7,11,12
Since the frequent use of doxorubicin-based, gemcitabine-based chemotherapy and pazopanib within this series, CCS is generally treated with conventional systemic therapies available for treatment of soft tissue sarcoma despite the poor evidence to support its use. All systemic therapies had a low response rate and a short mPFS. This is in line with other published cohorts.9 In a combined UK and US cohort of patients, there was a single response (n = 1/24) to doxorubicin in combination with ifosfamide and the mPFS was similarly short at 11 weeks.9 In our cohort, the highest response rate was seen with sunitinib. Small published cohorts13 and case reports14,15 have also supported the role of sunitinib in this population. The response rate seen for doxorubicin was similar to that for patients with unselected soft tissue sarcomas in the ANNOUNCE trial (ORR 14%, n = 36/258); however, the overall DCR was higher in ANNOUNCE (75.7%, n = 190/251) compared to 56% in our cohort. The mPFS for patients with CCS was shorter compared to the 6.8 months seen in ANNOUNCE.
Recent work by Schöffski et al. to profile CCS tumour samples from the CREATE trial has suggested alterations in five non-angiogenic-related pathways: PI3K-AKT, polymerase II transcription, DNA damage and mismatch repair, chromatin organization modifying enzymes and proteins involved in post-translational modification by small ubiquitin-like modifiers.8 Given the emerging evidence suggesting that epigenomic changes may play a key role in the oncogenesis of fusion-associated sarcomas, such as CCS,16 it is indeed intriguing that alterations in the CREATE cohort were seen related to epigenomic modifications. Drug treatments targeting such epigenomic changes, such as tazemetostat,17 are largely unexplored in CCS but may warrant further investigations. There are currently no actively recruiting clinical trials exploring the use of agents which target epigenomic alterations. There are currently no actively recruiting clinical trials exploring the use of agents which target epigenomic alterations. Beyond trials of immunotherapy, a phase I trial of anti-mitochondrial drug devimistat plus hydroxychloroquine (NCT04593758) was conducted.18 Given the aggressive clinical behaviour of CCS, it may be that a combination approach with epigenomic modifying drugs with chemotherapy may improve typically poor responses to cytotoxic sarcoma-type chemotherapy.
Given the short mPFS and low clinical benefit rate with sarcoma-type chemotherapy, an informed patient–clinician discussion is required to consider the use of standard cytotoxic chemotherapy. There has been interest in exploring the role of immunotherapy in the treatment of patients with CCS. In the phase IB portion of the IMMUNOSARC trial, there were two partial responses (n = 2/4) to nivolumab and sunitinib; however, the responses were of short duration.19 In the phase II OSCAR study, ORR was 7.1% in a combined cohort of patients with CCS and alveolar soft part sarcoma.20 Results of two additional ongoing phase II trials using dostarlimab (NCT04274023)21 and atezolizumab (NCT04458922)22 are expected in 2024. Results of these sarcoma-type-specific trials are awaited to further define the role of immune checkpoint inhibitors in this population.
Based on the results from our cohort, we advocate that CCS patients should be considered for early access to phase I trials. Given its ultra-rarity, rational design of prospective registries may be required to generate high-quality outcome-focused data to better understand the treatment and outcome of patients with CCS. If there is no clinical trial available, our data support the use of sunitinib first-line therapy in this population. We also advocate for tumour sequencing for patients with CCS in the hope of identifying novel treatment options. The decision to use cytotoxic chemotherapy requires a careful informed discussion between the patient and their oncology care team.
Our study has limitations that accompany all the retrospective cohorts. However, all patients had molecular confirmation of the diagnosis, which strengthens the results. All patients were treated at high-volume sarcoma centres within the WSN, with the expectation that similar standards of care were employed. The toxicity of treatment was not collected as this is difficult to extract retrospectively. The effect of radiotherapy and other local treatments was not explored in this cohort, and the impact of these treatments on the outcome of patients is not thoroughly known.
Conclusion
CCS is an ultra-rare sarcoma with a poor prognosis. In our series, response rate and mPFS with different systemic treatments were low. Sunitinib did demonstrate modest effectiveness compared to other therapies; however, sunitinib is not formally approved for the treatment of soft tissue sarcomas. Novel therapies for CCS are needed and the results of phase II studies with immune checkpoint inhibitors are awaited; however, it may be that epigenomic changes play a role in the oncogenesis of CCS. Prospective, translational registries are needed to further improve outcomes for this challenging population.
Acknowledgements
The article is dedicated to the memory of Annabelle Mahar. We thank Ms Stephanie Elston for her invaluable help with administrative aspects of this multi-institution retrospective study.
Funding
None declared.
Disclosure
AS has received consulting fees from Merck and Medison. RLJ is the recipient of grants/research support from MSD and GSK and is the recipient of consultation fees from Adaptimmune, Athenex, Blueprint, Clinigen, Eisai, Epizyme, Daichii, Deciphera, Immunedesign, Lilly, Merck, Pharmamar, and UptoDate. PR has received honoraria for lectures and advisory boards from BMS, MSD, Novartis, Pierre Fabre, Sanofi, Merck, Philogen, and Blueprint Medicines outside of the scope of the trial. AMC has received honoraria as invited speaker from BMS, MSD, Novartis, and Pierre Fabre. FD has received honoraria from PharmaMar, GSK, and Bayer, and travel grants from PharmaMar. GGB has received honoraria from Eli Lilly, Eisai, PharmaMar, GSK, and MSD; travel grants from PharmaMar, Pfizer, and Eli Lilly; and personal fees from AboutEvents, EditaMed, and Eli Lilly. All other authors have declared no conflicts of interest.
References
- 1.Hocar O., Le Cesne A., Berissi S., et al. Clear cell sarcoma (malignant melanoma) of soft parts: a clinicopathologic study of 52 cases. Dermatol Res Pract. 2012;2012 doi: 10.1155/2012/984096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang W.-L., Mayordomo E., Zhang W., et al. Detection and characterization of EWSR1/ATF1 and EWSR1/CREB1 chimeric transcripts in clear cell sarcoma (melanoma of soft parts) Mod Pathol. 2009;22(9):1201–1209. doi: 10.1038/modpathol.2009.85. [DOI] [PubMed] [Google Scholar]
- 3.Gonzaga M.I., Grant L., Curtin C., Gootee J., Silberstein P., Voth E. The epidemiology and survivorship of clear cell sarcoma: a National Cancer Database (NCDB) review. J Cancer Res Clin Oncol. 2018;144(9):1711–1716. doi: 10.1007/s00432-018-2693-6. [DOI] [PubMed] [Google Scholar]
- 4.Thway K., Fisher C. Tumors with EWSR1-CREB1 and EWSR1-ATF1 fusions: the current status. Am J Surg Pathol. 2012;36(7):e1–e11. doi: 10.1097/PAS.0b013e31825485c5. [DOI] [PubMed] [Google Scholar]
- 5.Wagner A.J., Goldberg J.M., DuBois S.G., et al. Tivantinib (ARQ 197), a selective inhibitor of MET, in patients with microphthalmia transcription factor–associated tumors: results of a multicenter phase 2 trial. Cancer. 2012;118(23):5894–5902. doi: 10.1002/cncr.27582. [DOI] [PubMed] [Google Scholar]
- 6.Davis I.J., McFadden A.W., Zhang Y., et al. Identification of the receptor tyrosine kinase c-Met and its ligand, hepatocyte growth factor, as therapeutic targets in clear cell sarcoma. Cancer Res. 2010;70(2):639–645. doi: 10.1158/0008-5472.CAN-09-1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schöffski P., Wozniak A., Stacchiotti S., et al. Activity and safety of crizotinib in patients with advanced clear-cell sarcoma with MET alterations: European Organization for Research and Treatment of Cancer phase II trial 90101 ‘CREATE’. Ann Oncol. 2017;28(12):3000–3008. doi: 10.1093/annonc/mdx527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee C.-J., Modave E., Boeckx B., et al. Histopathological and molecular profiling of clear cell sarcoma and correlation with response to crizotinib: an exploratory study related to EORTC 90101 “CREATE” trial. Cancers. 2021;13(23):6057. doi: 10.3390/cancers13236057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jones R.L., Constantinidou A., Thway K., et al. Chemotherapy in clear cell sarcoma. Med Oncol. 2011;28(3):859–863. doi: 10.1007/s12032-010-9502-7. [DOI] [PubMed] [Google Scholar]
- 10.Stacchiotti S., Frezza A.M., Blay J.Y., et al. Ultra-rare sarcomas: a consensus paper from the Connective Tissue Oncology Society community of experts on the incidence threshold and the list of entities. Cancer. 2021;127(16):2934–2942. doi: 10.1002/cncr.33618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kawai A., Hosono A., Nakayama R., et al. Clear cell sarcoma of tendons and aponeuroses: a study of 75 patients. Cancer. 2007;109(1):109–116. doi: 10.1002/cncr.22380. [DOI] [PubMed] [Google Scholar]
- 12.Bianchi G., Charoenlap C., Cocchi S., et al. Clear cell sarcoma of soft tissue: a retrospective review and analysis of 31 cases treated at Istituto Ortopedico Rizzoli. Eur J Surg Oncol. 2014;40(5):505–510. doi: 10.1016/j.ejso.2014.01.016. [DOI] [PubMed] [Google Scholar]
- 13.Stacchiotti S., Palassini E., Negri T., et al. Sunitinib malate in clear cell sarcoma. Ann Oncol. 2012;23:ix484. doi: 10.1093/annonc/mdp611. [DOI] [PubMed] [Google Scholar]
- 14.Tazzari M., Palassini E., Vergani B., et al. Melan-A/MART-1 immunity in a EWS-ATF1 translocated clear cell sarcoma patient treated with sunitinib: a case report. BMC Cancer. 2015;15(1):1–8. doi: 10.1186/s12885-015-1044-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stacchiotti S., Grosso F., Negri T., et al. Tumor response to sunitinib malate observed in clear-cell sarcoma. Ann Oncol. 2010;21(5):1130–1131. doi: 10.1093/annonc/mdp611. [DOI] [PubMed] [Google Scholar]
- 16.Nacev B.A., Jones K.B., Intlekofer A.M., et al. The epigenomics of sarcoma. Nat Rev Cancer. 2020;20(10):608–623. doi: 10.1038/s41568-020-0288-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gounder M., Schöffski P., Jones R.L., et al. Tazemetostat in advanced epithelioid sarcoma with loss of INI1/SMARCB1: an international, open-label, phase 2 basket study. Lancet Oncol. 2020;21(11):1423–1432. doi: 10.1016/S1470-2045(20)30451-4. [DOI] [PubMed] [Google Scholar]
- 18.ClinicalTrials.gov. NCT04593758 To Evaluate Maximally Tolerated Dose (MTD), Safety and Efficacy of CPI-613® (Devimistat) Plus Hydroxychloroquine in Patients With Relapsed or Refractory Clear Cell Sarcoma of Soft Tissue: NIH US National Library of Medicine; 2022. Available at https://clinicaltrials.gov/ct2/show/NCT04593758. Accessed February 2, 2022.
- 19.Martin-Broto J., Hindi N., Grignani G., et al. Nivolumab and sunitinib combination in advanced soft tissue sarcomas: a multicenter, single-arm, phase Ib/II trial. J Immunother Cancer. 2020;8(2) doi: 10.1136/jitc-2020-001561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kawai A., Nishikawa T., Okamura N., Shibata T., Tamaura K., Ueda G. Canada: CTOS; Vancouver, BC: 2020. Efficacy and Safety of Nivolumab Monotherapy in Patients with Unresectable Clear Cell Sarcoma and Alveolar Soft Part Sarcoma (OSCAR TRIAL, NCCH1510): A Multicenter, Phase 2 Clinical Trial. [Google Scholar]
- 21.ClinicalTrials.gov. NCT04274023: Study on TSR-042 in Advanced Clear Cell Sarcoma (ACCeSs): NIH US National Library of Medicine. 2022. Available at https://clinicaltrials.gov/ct2/show/NCT04274023?cond=clear+cell+sarcoma&draw=2&rank=2. Accessed February 2, 2022.
- 22.ClinicalTrials.gov. NCT04458922: Testing Atezolizumab in Patients >= 2 Years Old With Newly Diagnosed, Unresectable, or Metastatic Clear Cell Sarcoma or Chondrosarcoma: NIH U.S. National Library of Medicine. 2022. Available at https://clinicaltrials.gov/ct2/show/NCT04458922?cond=clear+cell+sarcoma&draw=2&rank=3. Accessed February 2, 2022.