Abstract
Background
KRAS gene mutations can predict prognosis and treatment response in patients with metastatic colorectal cancer (mCRC).
Methods
We undertook a meta-analysis of three randomized, placebo-controlled trials (RECOURSE, TERRA and J003) to investigate the impact of KRAS mutations in codons 12 or 13 on overall survival (OS) and progression-free survival in patients receiving trifluridine/tipiracil (FTD/TPI) for refractory mCRC.
Results
A total of 1375 patients were included, of whom 478 had a KRAS codon 12 mutation and 130 had a KRAS codon 13 mutation. In univariate analyses, the absence of a KRAS codon 12 mutation was found to significantly increase the OS benefit of FTD/TPI relative to placebo compared with the presence of the mutation {hazard ratio (HR), 0.62 [95% confidence interval (CI): 0.53-0.72] versus 0.86 (0.70-1.05), respectively; interaction P = 0.0206}. Multivariate analyses showed that taking confounding factors into account reduced the difference in treatment effect between the presence and the absence of KRAS codon 12 mutations, confirming that treatment benefit was maintained in patients with [HR, 0.73 (95% CI: 0.59-0.89)] and without [HR, 0.63 (95% CI: 0.54-0.74)] codon 12 mutations (interaction P = 0.2939). KRAS mutations in codon 13 did not reduce the OS benefit of FTD/TPI relative to placebo, and, furthermore, KRAS mutations at either codon 12 or codon 13 did not affect the progression-free survival benefit.
Conclusions
Treatment with FTD/TPI produced a survival benefit, relative to placebo, regardless of KRAS codon 12 or 13 mutation status in patients with previously treated mCRC.
Key words: metastatic colorectal cancer, trifluridine/tipiracil, KRAS, survival, meta-analysis
Highlights
-
●
KRAS mutations are associated with negative outcomes in patients with mCRC; codon 12 and 13 mutations are the most common.
-
●
FTD/TPI was associated with longer median overall survival vs placebo both in patients with wild-type KRAS and mutant KRAS.
-
●
FTD/TPI produced a survival benefit, relative to placebo, regardless of KRAS codon 12 or 13 mutation status in this patient group.
Introduction
In patients with metastatic colorectal cancer (mCRC), KRAS mutations are associated with several negative outcomes.1, 2, 3 Patients with colorectal cancer who have a mutant KRAS gene have a significantly higher incidence of lung, bone and brain metastases compared with patients who have wild-type KRAS.1 In addition, the presence of mutant KRAS in patients with mCRC predicts a lack of response to drugs targeting epidermal growth factor receptors (anti-EGFRs), but does not preclude benefit from oxaliplatin or irinotecan.2,4
Codons 12 and 13 in exon 2 are the most common sites of KRAS gene mutation in patients with colorectal cancer, being found in ∼40% of patients with the disease.5 Patients with KRAS codon 12 mutations have been reported to have poor overall survival (OS).6,7 In contrast, the prognostic value of KRAS codon 13 mutations is controversial.8, 9, 10
Therapeutic options for patients with mCRC and KRAS codon 12 or 13 mutations are limited.2 Trifluridine/tipiracil (FTD/TPI) is approved for the treatment of patients with mCRC who have been previously treated with, or are not considered to be candidates for, fluoropyrimidine-, oxaliplatin- and irinotecan-based chemotherapies, anti-vascular endothelial growth factor therapy or anti-EGFR therapy.11, 12, 13
In the phase III RECOURSE trial, which included patients with refractory mCRC (n = 800), median OS was significantly longer in patients randomized to FTD/TPI than to placebo {7.1 versus 5.3 months; hazard ratio (HR), 0.68 [95% confidence interval (CI): 0.58-0.81]; P < 0.001}.14 There was no significant difference in OS in the cohort of 407 patients (51%) with mutant KRAS [HR, 0.80 (95% CI: 0.63-1.02)], justifying further study in a larger patient population by including patients from other studies and increasing the study population to 708 patients with mutant KRAS colorectal cancer (478 with KRAS codon 12 mutations and 130 with KRAS codon 13 mutations). At present, however, there are insufficient data to guide the use of FTD/TPI in patients with previously treated mCRC in whom KRAS codon 12 or 13 mutation status is known.
Therefore, we undertook a meta-analysis to determine whether the presence of mutations at KRAS codon 12 or 13 impacts the efficacy of FTD/TPI, in terms of survival benefits, in patients with previously treated mCRC.
Materials and methods
Included studies
We carried out a meta-analysis of data from three randomized, placebo-controlled trials of FTD/TPI in patients with previously treated mCRC in whom KRAS codon 12 and 13 mutation status was known. The three trials were: the phase III RECOURSE trial (NCT01607957),14 the phase III TERRA trial (NCT01955837)15 and the phase II J003 trial (JapicCTI-090880).16 The most up-to-date OS data were used for two of the trials (RECOURSE17 and J00318).
Data extraction
For each of the three studies, the following data were extracted from the intent-to-treat populations: number of patients; KRAS codon 12 and 13 mutation status; baseline characteristics [age, sex, race, region, Eastern Cooperative Oncology Group (ECOG) performance status, neutrophil-to-lymphocyte ratio, number of metastatic sites, number of previous treatment regimens, primary diagnosis (colon or rectum), prior use of regorafenib, time to first metastasis, and BRAF mutation status]; OS; and progression-free survival (PFS).
Study endpoints
For this study, survival endpoints (OS and PFS) were selected as the most important measures of efficacy. OS was defined as the time from randomization to death from any cause, whereas PFS was defined as the time from randomization to either disease progression or death from any cause.
Statistical analysis
The objective of the analysis was to investigate whether KRAS mutations can be considered as a predictive factor. For this reason, the statistical interaction between KRAS mutations and treatment arm was investigated for both OS and PFS. This was done with a meta-analysis of three trials based on individual study data, including univariate and multivariate analyses, to test for statistical interaction according to methods described by Katsahian et al.19 Between-study heterogeneity in treatment effect was assessed using the heterogeneity test.20 Since no between-study heterogeneity in terms of treatment effect was demonstrated, a fixed-effect model with a study level stratified approach was used for the meta-analysis.
Univariate analyses based on Cox proportional hazards models were used to calculate HRs and corresponding 95% CIs for both OS and PFS across the different subgroups of KRAS mutation status. OS and PFS by treatment arm were also estimated using Kaplan–Meier curves for each mutation status subgroup, and further characterized in terms of the median with the corresponding two-sided 95% CI. P values were based on the log-rank test, and P values for interaction between mutation status and treatment effect were based on the Wald test.
A multivariate analysis based on a Cox proportional hazards model was carried out for the study level stratified meta-analysis using individual patient data. A stepwise selection process (including mutation status at each step and retaining factors that were significant at the 10% level) was used to account for confounding factors. The covariates submitted to the model were: time since metastasis (<18 months versus ≥18 months versus unknown); ECOG performance status (0 versus ≥1); number of metastatic sites (as judged by the investigator; 1-2 versus ≥3); neutrophil-to-lymphocyte ratio (<3 versus ≥3); primary diagnosis (colon versus rectum); region (Asia versus other); number of previous treatment regimens (2 versus 3 versus ≥4); sex (male versus female); age (<65 years versus ≥65 years); prior use of regorafenib (yes versus no). Once the final subset of significant covariates was established, treatment and interaction between treatment and mutation status were added to the model to assess their effects. The HR for FTD/TPI relative to placebo was reported with its 95% CI as well as the HR and 95% CI for all factors retained by the stepwise procedure. P values were calculated using the Wald chi-square test.
All statistical analyses were carried out using SAS 9.4 (SAS Institute, Cary, NC) and R software 3.6.1 (R foundation, Vienna, Austria).21
Sensitivity analysis
Sensitivity analyses were conducted using data from the RECOURSE and TERRA trials only. For the J003 trial, information on methodology used was not sufficiently detailed to support the use of different approaches. OS data from these trials were re-analyzed using two alternative ways of determining codon mutation status: a ‘method-dependent’ approach, and a ‘strict’ approach. It should be noted that according to the literature and expert opinion, only some methods of measurement can differentiate between mutations in codons 12 and 13. Methods that allow discrimination between KRAS codon 12 and KRAS codon 13 are: allele-specific oligonucleotide probing; amplification-refractory mutation system (ARMS); direct sequencing; DxS Scorpion Technology; Scorpion ARMS; pyrosequencing; restriction endonuclease-mediated selective PCR (REMS-PCR); and cycleave PCR.
In the ‘method-dependent’ analysis, patients were excluded if the KRAS status was unclear (i.e. the precise mutation was not reported, or indicated the presence of mutations in both codon 12 and codon 13) and the method used was unable to differentiate between codons 12 and 13. In the ‘strict’ approach, patients were excluded if the KRAS status was unclear regardless of the analytic method used.
Results
Study population
The meta-analysis included data from 1375 patients who participated in the J003, RECOURSE and TERRA trials. Baseline demographics and disease characteristics according to KRAS codon 12 mutation status are shown in Table 1 (for individual trial data, see Supplementary Tables S1 and S2, available at 10.1016/j.esmoop.2022.100511). In total, 917 patients were randomized to FTD/TPI and 458 patients were randomized to placebo; 736 patients had wild-type KRAS (492 FTD/TPI, 244 placebo) and 635 patients had mutant KRAS (422 FTD/TPI, 213 placebo). KRAS status was not known in four patients (all from the J003 trial). Among patients who received FTD/TPI, codon 12 mutations were present in 313 patients and absent in 604 patients, whereas codon 13 mutations were present in 85 patients and absent in 832 patients. Overall, KRAS codon 12 mutations were present in 34.8% (n = 478) of patients.
Table 1.
Baseline demographics and disease characteristics for all patients in the meta-analysis, according to KRAS codon 12 mutation status (yes = present; no = absent) and assigned study treatment (FTD/TPI or placebo). Continuous variables are expressed as mean (SD). Categorical variables are expressed as number (%)
|
KRAS codon 12 mutation status |
No |
Yes |
||
|---|---|---|---|---|
| Treatment arm | FTD/TPI (n = 604) | Placebo (n = 293) | FTD/TPI (n = 313) | Placebo (n = 165) |
| Age, years | 60.3 (10.8) | 60.1 (10.5) | 59.6 (10.2) | 59.1 (12.1) |
| Age | ||||
| <65 years | 365 (60.4) | 184 (62.8) | 201 (64.2) | 101 (61.2) |
| ≥65 years | 239 (39.6) | 109 (37.2) | 112 (35.8) | 64 (38.8) |
| Male | 381 (63.1) | 185 (63.1) | 179 (57.2) | 92 (55.8) |
| Race | ||||
| Asian | 388 (64.2) | 178 (60.8) | 179 (57.2) | 108 (65.5) |
| Black/African-American | 2 (0.3) | 1 (0.3) | 2 (0.6) | 4 (2.4) |
| Caucasian | 188 (31.1) | 106 (36.2) | 118 (37.7) | 49 (29.7) |
| Not collected | 26 (4.3) | 8 (2.7) | 14 (4.5) | 4 (2.4) |
| Region | ||||
| Asia | 385 (63.7) | 175 (59.7) | 176 (56.2) | 105 (63.6) |
| Westerna | 219 (36.3) | 118 (40.3) | 137 (43.8) | 60 (36.4) |
| ECOG performance status | ||||
| 0 | 279 (46.2) | 127 (43.3) | 158 (50.5) | 85 (51.5) |
| ≥1 | 325 (53.8) | 166 (56.7) | 155 (49.5) | 80 (48.5) |
| Neutrophil-to-lymphocyte ratio | ||||
| n | 595 | 290 | 308 | 165 |
| <3 | 279 (46.9) | 127 (43.8) | 119 (38.6) | 83 (50.3) |
| ≥3 | 316 (53.1) | 163 (56.2) | 189 (61.4) | 82 (49.7) |
| Number of metastatic sites | ||||
| 1-2 | 381 (63.1) | 160 (54.6) | 177 (56.5) | 106 (64.2) |
| ≥3 | 223 (36.9) | 133 (45.4) | 136 (43.5) | 59 (35.7) |
| Number of prior treatment regimens | ||||
| 2 | 91 (15.1) | 36 (12.3) | 83 (26.5) | 47 (28.5) |
| 3 | 150 (24.8) | 67 (22.9) | 89 (28.4) | 39 (23.6) |
| ≥4 | 362 (59.9) | 190 (64.8) | 139 (44.4) | 79 (47.9) |
| Primary diagnosis | ||||
| Colon | 358 (59.3) | 184 (62.8) | 197 (62.9) | 98 (59.4) |
| Rectum | 246 (40.7) | 109 (37.2) | 116 (37.1) | 67 (40.6) |
| Prior use of regorafenib | ||||
| No | 547 (90.6) | 253 (86.3) | 274 (87.5) | 151 (91.5) |
| Time since first metastasis, months | 32.6 (22.2) | 35.6 (22.0) | 27.2 (17.3) | 28.0 (16.5) |
| Time since first metastasis by class | ||||
| <18 months | 143 (23.7) | 56 (19.1) | 98 (31.3) | 50 (30.3) |
| ≥18 months | 383 (63.4) | 203 (69.3) | 181 (57.8) | 92 (55.8) |
| Unknown | 78 (12.9) | 34 (11.6) | 34 (10.9) | 23 (13.9) |
| BRAF mutation status | ||||
| Wild-type | 116 (19.2) | 62 (21.2) | 56 (17.9) | 29 (17.6) |
| Mutant | 9 (1.5) | 6 (2.0) | 1 (0.3) | 1 (0.6) |
| Unknown | 479 (79.3) | 225 (76.8) | 256 (81.8) | 135 (81.8) |
ECOG, Eastern Cooperative Oncology Group; FTD/TPI, trifluridine/tipiracil; SD, standard deviation.
USA, Europe and Australia.
Analysis groups were generally well balanced with respect to baseline characteristics (Table 1), with most patients being male, aged <65 years, and of Asian ethnicity.
Wild-type versus mutant KRAS
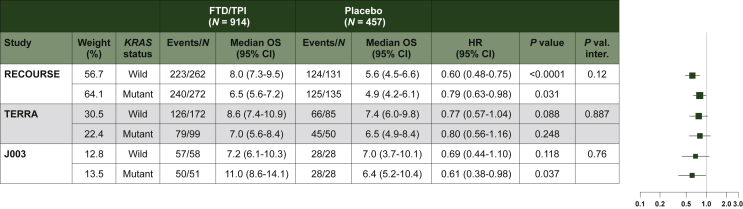
In the meta-analysis of individual patient data, FTD/TPI was associated with a statistically significant OS prolongation compared with placebo, both in patients with wild-type KRAS and with mutant KRAS [Figure 1; wild-type: HR, 0.66 (95% CI: 0.56-0.78) P < 0.0001; mutant: HR, 0.77 (95% CI: 0.64-0.91) P = 0.0026. For more details see Supplementary Figure S1A, available at 10.1016/j.esmoop.2022.100511].
Figure 1.
Overall survival according to KRASmutation status.
P value: P value from log-rank test. HR (FTD/TPI versus placebo) and 95% CI obtained from a distinct unstratified Cox model for each subgroup. P val. inter: P value from unstratified Cox model including arm treatment, subgroup and their interaction in the model. Ties handling method: Efron.
CI, confidence interval; FTD/TPI, trifluridine/tipiracil; HR, hazard ratio; OS, overall survival.
Codon 12 mutation status
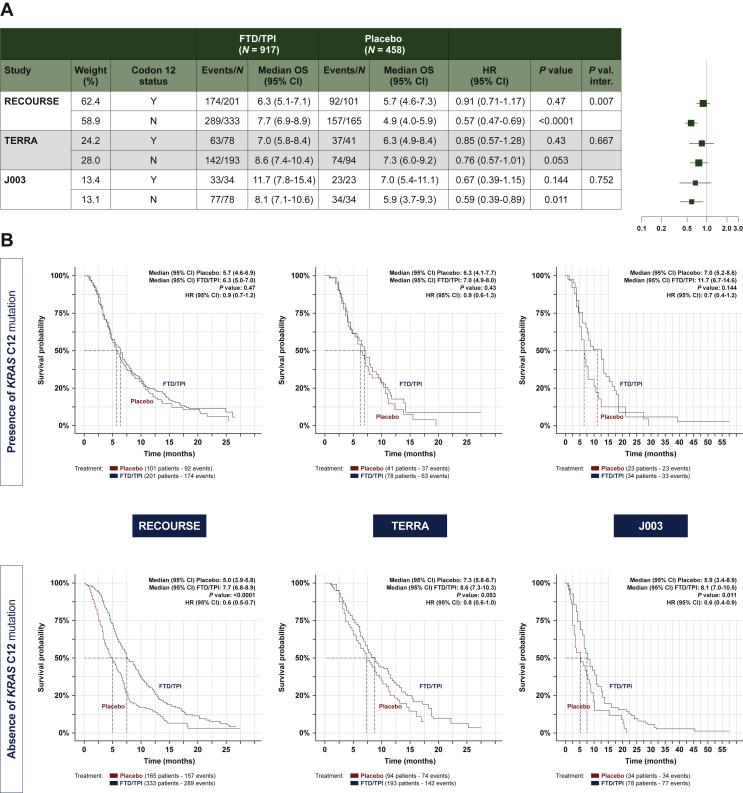
In meta-analysis of individual data from all three studies, a non-statistically significant prolongation of the OS of FTD/TPI was observed in patients with [HR, 0.86 (95% CI: 0.70-1.05) P = 0.1385] and statistical significant prolongation was observed in patients without [HR, 0.62 (95% CI: 0.53-0.72) P < 0.0001] KRAS codon 12 mutations (Table 2, Figure 2 and Supplementary Figure S1B, available at 10.1016/j.esmoop.2022.100511), although the effect size was more pronounced in the latter subgroup (interaction P = 0.0206). For more details see Supplementary Figure S1B, available at 10.1016/j.esmoop.2022.100511.
Table 2.
Meta-analysis based on stratified, fixed-effects Cox model analysis of overall survival in patients receiving trifluridine/tipiracil (FTD/TPI) or placebo, according to KRAS codon 12 and 13 mutation status
| KRAS mutation | FTD/TPI versus placeboa |
Interaction P valueb |
||
|---|---|---|---|---|
| HR | 95% CI | P value | ||
| Codon 12 | ||||
| Yes | 0.86 | 0.70-1.05 | 0.1385 | 0.0206 |
| No | 0.62 | 0.53-0.72 | <0.0001 | |
| Codon 13 | ||||
| Yes | 0.46 | 0.31-0.69 | 0.0001 | 0.0104 |
| No | 0.74 | 0.65-0.84 | <0.0001 | |
CI, confidence interval; HR, hazard ratio.
Stratified Cox model based on study level.
Interaction between treatment arm and KRAS codon 12 or 13 mutation status (stratified Cox model based on study level).
Figure 2.
Overall survival according to KRAScodon 12 mutation status. (A) Forest plot. (B) Kaplan–Meier curves.
P value: P value from log-rank test. HR (FTD/TPI versus placebo) and 95% CI obtained from a distinct unstratified Cox model for each subgroup. P val. inter: P value from unstratified Cox model including arm treatment, subgroup and their interaction in the model. Ties handling method: Efron.
CI, confidence interval; FTD/TPI, trifluridine/tipiracil; HR, hazard ratio; OS, overall survival.
FTD/TPI was associated with an OS benefit, versus placebo, in patients without a mutation in KRAS codon 12 in each of the individual trials [RECOURSE: HR, 0.57 (95% CI: 0.47-0.69); J003: HR, 0.59 (95% CI: 0.39-0.89); TERRA: HR, 0.76 (95% CI: 0.57-1.01)]. In patients with a mutation in KRAS codon 12, an OS benefit of FTD/TPI over placebo was present but less evident in each of the individual trials. A significant quantitative interaction between treatment effect and KRAS codon 12 mutation status was detected in the RECOURSE trial [HR, 0.91 (95% CI: 0.71-1.17); interaction P = 0.007] but not in the TERRA [HR, 0.85 (95% CI: 0.57-1.28); interaction P = 0.667] or J003 [HR, 0.67 (95% CI: 0.39-1.15); P = 0.752] trials (Figure 2).
In the multivariate analysis (Table 3), OS was longer with FTD/TPI than with placebo regardless of codon 12 status [with codon 12 mutation: HR, 0.73 (95% CI: 0.59-0.89); without codon 12 mutation: HR, 0.63 (95% CI: 0.54-0.74)]; no interaction between treatment effect and KRAS codon 12 mutation status was detected (P = 0.2939). Prognostic factors retained in the multivariate analysis of OS were number of metastases, neutrophil-to-lymphocyte ratio, time since metastasis, ECOG performance status and number of prior treatment regimens.
Table 3.
Multivariate analysis of overall survival
| Factor | Codon 12 mutation | Comparison | HRa | 95% CIa | P valueb | Interaction P valuec | Missing values (n) |
|---|---|---|---|---|---|---|---|
| Treatment effect | — | FTD/TPI versus placebo | 0.68 | 0.60-0.77 | <0.0001 | 0 | |
| Treatment effect by codon 12 mutation statusd | Yes No |
FTD/TPI versus placebo FTD/TPI versus placebo |
0.73 0.63 |
0.59-0.89 0.54-0.74 |
0.0018 <0.0001 |
0.2939 | 0 |
| Number of metastases | — | 1-2 versus ≥3 | 0.56 | 0.50-0.63 | <0.0001 | 0 | |
| Neutrophils-lymphocytes ratio | — | <3 versus ≥3 | 0.56 | 0.49-0.63 | <0.0001 | 17 | |
| Time since metastasis (months) | — | <18 versus ≥18 | 0.66 | 0.56-0.77 | <0.0001 | 0 | |
| ECOG performance status | — | 0 versus ≥1 | 0.74 | 0.65-0.84 | <0.0001 | 0 | |
| Number of prior treatment regimens | — | 2 versus ≥4 3 versus ≥4 |
1.20 1.15 |
1.01-1.43 1.00-1.33 |
0.0340 0.0524 |
3 |
CI, confidence interval; ECOG, Eastern Cooperative Oncology Group; FTD/TPI, trifluridine/tipiracil; HR, hazard ratio.
HR and 95% CI from the full Cox regression model, stratified by study, and including terms for treatment arm, codon 12 mutation status, interaction between treatment arm and codon 12 mutation status; all factors were retained from stepwise selection (entry and stay α = 0.1).
Wald chi-square test.
P value for interaction with treatment arm from the full model, plus the two-way interaction with only the factor shown (i.e. separate models including only one factor crossed with treatment).
For interaction factor, the HR corresponds to the treatment effect adjusted to all significant factors.
Regardless of codon 12 mutation status, PFS was significantly longer with FTD/TPI than with placebo in each individual trial and in the meta-analysis of all three trials (see Supplementary Figure S2A and B, available at 10.1016/j.esmoop.2022.100511).
Codon 13 mutation status
There were 130 patients (9.5%) with and 1245 patients (90.5%) without a KRAS codon 13 mutation across the three trials.
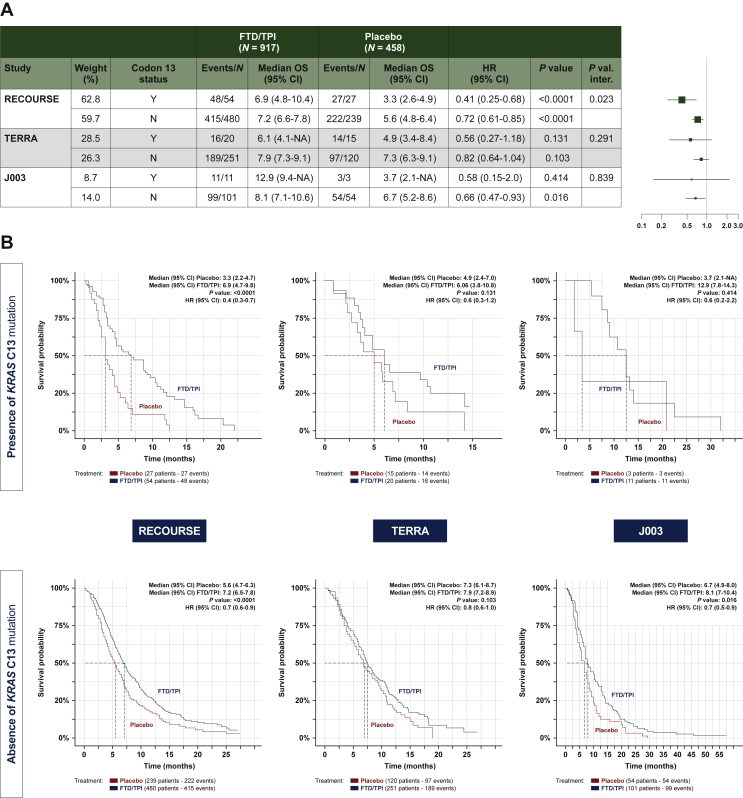
In the meta-analysis of individual data, there is a statistically significant OS prolongation for patients with FTD/TPI compared with placebo both in patients with [HR, 0.46 (95% CI: 0.31-0.69) P = 0.0001] and without [HR, 0.74 (95% CI: 0.65-0.84) P < 0.0001] KRAS codon 13 mutations (Table 2 and Figure 3) For more details see Supplementary Figure S1B, available at 10.1016/j.esmoop.2022.100511. There was a significant interaction between treatment arm and KRAS codon 13 mutation status (P = 0.0104). In the three individual trials, FTD/TPI was associated with longer median OS than placebo regardless of codon 13 mutation status; however, between-group differences did not consistently show statistical significance (Figure 3). In the TERRA and J003 trials, codon 13 mutation status did not significantly alter the effect of FTD/TPI on OS, relative to placebo; however, a difference in treatment effect was detected between those with and without the codon 13 mutation in the RECOURSE trial (interaction P = 0.023). PFS was significantly longer with FTD/TPI than with placebo irrespective of codon 13 mutation status, both for the individual trials and in the meta-analysis (see Supplementary Figure S3A and B, available at 10.1016/j.esmoop.2022.100511).
Figure 3.
Overall survival according to KRAScodon 13 mutation status. (A) Forest plot. (B) Kaplan–Meier curves.
P value: P value from log-rank test. HR (FTD/TPI versus placebo) and 95% CI obtained from a distinct unstratified Cox model for each subgroup. P val. inter: P value from unstratified Cox model including arm treatment, subgroup and their interaction in the model. Ties handling method: Efron.
CI, confidence interval; FTD/TPI, trifluridine/tipiracil; HR, hazard ratio; OS, overall survival.
Sensitivity analysis
Similar results to the main analyses were obtained when the data from the RECOURSE and TERRA trials were re-analyzed using method-dependent and strict approaches to determining mutation status (see Supplementary Figure S4A-D, available at 10.1016/j.esmoop.2022.100511).
Discussion
This meta-analysis was conducted to determine the effect of KRAS codon 12 and 13 mutations on the efficacy of FTD/TPI in previously treated patients with mCRC. The analysis was carried out using data from the RECOURSE,14 TERRA15 and J003 trials.16 Although the results of the univariate analysis showed that the effect of FTD/TPI on OS, relative to placebo, was reduced in the presence of mutant KRAS codon 12, the multivariate analysis showed that treatment with FTD/TPI produced an OS benefit over placebo regardless of KRAS codon 12 mutation status. The significant interaction that we observed in the univariate analysis was strongly driven by the RECOURSE trial, since no effect of KRAS codon 12 mutation status was detected in the TERRA and J003 trials. With respect to PFS, FTD/TPI was associated with similar benefits in patients with and without mutations in KRAS codon 12.
KRAS mutations are reportedly associated with a poor response to treatment of mCRC.2,22 Mutations in KRAS codon 12 have been associated with poor outcomes and negatively affecting the antitumor effectiveness of some therapies in early mCRC.23,24 In contrast with previously published analyses,1,24 we were not able to confirm the prognostic value of KRAS codon 12 mutations using survival data from placebo recipients. In our multivariate analysis, a benefit of FTD/TPI on OS was shown in patients with and without KRAS codon 12 mutations.
In early mCRC, KRAS codon 13 mutations appear to predict worse OS in Chinese patients in comparison with wild-type KRAS, whereas KRAS codon 12 mutations do not.8 Little is known about the prognostic significance of KRAS mutations in later mCRC. Our analysis showed that survival outcomes were very poor in placebo-treated patients with KRAS codon 13 mutations, and that FTD/TPI was associated with a survival benefit regardless of KRAS codon 13 mutation status. Fewer than 10% of patients in the meta-analysis had mutant KRAS codon 13, however, and these findings should therefore be interpreted with caution.
Previous analyses of clinical trial data have found that the efficacy of FTD/TPI in mCRC is unaffected by KRAS mutation status.25, 26, 27 In both an exploratory analysis of the RECOURSE trial27 and a previously published meta-analysis of the RECOURSE, TERRA and J003 trials,25 OS was found to be similar in patients with wild-type and mutant KRAS. By contrast, a real-world study of patients with refractory mCRC treated with FTD/TPI as part of a compassionate use program found that the presence of a KRAS mutation was associated with shorter OS.26
Effective options for the third- or fourth-line treatment of mCRC are limited.2 The results of our meta-analysis therefore suggest that treatment with FTD/TPI is beneficial for patients with KRAS wild-type disease and in those with KRAS codon 12 or 13 mutations.
Our analysis has several limitations. First, only three trials were available for inclusion in the meta-analysis, which has implications for the strength of the findings, particularly with respect to codon 13 mutations; additionally, most of the patients came from one of the trials (RECOURSE). Second, the individual trials were not designed to assess the effect of KRAS codon 12 and 13 mutations on treatment effects. Third, data on specific KRAS point mutations were not available for all patients. Fourth, only one strategy of covariate selection was used for the multivariate model (stepwise approach is a standard practice in clinical trial). Fifth, our analysis was limited to KRAS mutational status, meaning that the NRAS status was not examined.
Accordingly, our findings require confirmation, in prospective controlled trials and real-world studies, before definitive conclusions about the effects of KRAS codon 12 and 13 mutations on the effectiveness of FTD/TPI can be made.
In conclusion, this meta-analysis of three randomized, placebo-controlled trials provides preliminary evidence that treatment with FTD/TPI produced a survival benefit, relative to placebo, regardless of KRAS codon 12 or 13 mutation status in patients with previously treated mCRC.
Acknowledgements
We thank Georgii Filatov and Richard Crampton of Springer Healthcare Communications, who wrote the outline and subsequent drafts of this manuscript. This medical writing assistance was funded by Servier.
Funding
This work was supported by Institut de Recherches Internationales Servier, France (no grant number).
Disclosure
TY has received honoraria for speaking engagements from Taiho, Chugai, Eli Lilly, Sanofi, Takeda, Merck Biopharma and Bayer; and research expenses from Taiho, Sumitomo Dainippon, Ono, Chugai, Amgen, Parexel International, Merck Sharp & Dohme (MSD), Daiichi Sankyo and Sanofi. EVC has participated in advisory boards for Array, Astellas, AstraZeneca, Bayer, BeiGene, Biocartis, Boehringer Ingelheim, Bristol Myers Squibb, Celgene, Daiichi, Halozyme, GlaxoSmithKline, Incyte, Ipsen, Lilly, MSD, Merck KGaA, Novartis, Pierre Fabre, Roche, Servier, Sirtex and Taiho, and has received research grants (paid to his institution) from Amgen, Bayer, Boehringer Ingelheim, Bristol Myers Squibb, Celgene, Ipsen, Lilly, MSD, Merck KGaA, Novartis, Roche and Servier. JL has received honoraria for speaking engagements from Henrui, Eli Lilly, Sanofi, Merck, Bayer and Taiho; and research funding from Taiho, Merck, MSD and Henrui. LS, VS and AO have received financial support for their research from Taiho via their respective institutions. LX, PA, RF, VC and NA are employees of Servier. All other authors have declared no conflicts of interest.
Supplementary data
P value: P value from log-rank test. HR (FTD/TPI versus placebo) and 95% CI obtained from a distinct unstratified Cox model for each subgroup. P val. inter: P value from unstratified Cox model including arm treatment, subgroup and their interaction in the model. Ties handling method: Efron.
CI, confidence interval; FTD/TPI, trifluridine/tipiracil; HR, hazard ratio; OS, overall survival.
References
- 1.Yaeger R., Cowell E., Chou J.F., et al. RAS mutations affect pattern of metastatic spread and increase propensity for brain metastasis in colorectal cancer. Cancer. 2015;121(8):1195–1203. doi: 10.1002/cncr.29196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Van Cutsem E., Cervantes A., Adam R., et al. ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann Oncol. 2016;27(8):1386–1422. doi: 10.1093/annonc/mdw235. [DOI] [PubMed] [Google Scholar]
- 3.Modest D.P., Ricard I., Heinemann V., et al. Outcome according to KRAS-, NRAS- and BRAF-mutation as well as KRAS mutation variants: pooled analysis of five randomized trials in metastatic colorectal cancer by the AIO colorectal cancer study group. Ann Oncol. 2016;27(9):1746–1753. doi: 10.1093/annonc/mdw261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Richman S.D., Seymour M.T., Chambers P., et al. KRAS and BRAF mutations in advanced colorectal cancer are associated with poor prognosis but do not preclude benefit from oxaliplatin or irinotecan: results from the MRC FOCUS trial. J Clin Oncol. 2009;27(35):5931–5937. doi: 10.1200/JCO.2009.22.4295. [DOI] [PubMed] [Google Scholar]
- 5.Vaughn C.P., Zobell S.D., Furtado L.V., Baker C.L., Samowitz W.S. Frequency of KRAS, BRAF, and NRAS mutations in colorectal cancer. Genes Chromosomes Cancer. 2011;50(5):307–312. doi: 10.1002/gcc.20854. [DOI] [PubMed] [Google Scholar]
- 6.Jones R.P., Sutton P.A., Evans J.P., et al. Specific mutations in KRAS codon 12 are associated with worse overall survival in patients with advanced and recurrent colorectal cancer. Br J Cancer. 2017;116(7):923–929. doi: 10.1038/bjc.2017.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Margonis G.A., Kim Y., Spolverato G., et al. Association between specific mutations in KRAS codon 12 and colorectal liver metastasis. JAMA Surg. 2015;150(8):722–729. doi: 10.1001/jamasurg.2015.0313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen J., Guo F., Shi X., et al. BRAF V600E mutation and KRAS codon 13 mutations predict poor survival in Chinese colorectal cancer patients. BMC Cancer. 2014;14:802. doi: 10.1186/1471-2407-14-802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kwak M.S., Cha J.M., Yoon J.Y., et al. Prognostic value of KRAS codon 13 gene mutation for overall survival in colorectal cancer: Direct and indirect comparison meta-analysis. Medicine (Baltimore) 2017;96(35) doi: 10.1097/MD.0000000000007882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Renaud S., Guerrera F., Seitlinger J., et al. KRAS exon 2 codon 13 mutation is associated with a better prognosis than codon 12 mutation following lung metastasectomy in colorectal cancer. Oncotarget. 2017;8(2):2514–2524. doi: 10.18632/oncotarget.13697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pharmaceuticals and Medical Devices Agency (PMDA) 2014 18 February. Review report: Lonsurf. https://www.pmda.go.jp/files/000207987.pdf Available at.
- 12.U.S. Food and Drug Administration (FDA) 2015 16 February. Lonsurf: Prescribing information. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/207981s009lbl.pdf Available at.
- 13.European Medicines Agency (EMA) 2016 16 February. Lonsurf: EPAR - Product information. https://www.ema.europa.eu/en/documents/product-information/lonsurf-epar-product-information_en.pdf Available at.
- 14.Mayer R.J., Van Cutsem E., Falcone A., et al. Randomized trial of TAS-102 for refractory metastatic colorectal cancer. N Engl J Med. 2015;372(20):1909–1919. doi: 10.1056/NEJMoa1414325. [DOI] [PubMed] [Google Scholar]
- 15.Xu J., Kim T.W., Shen L., et al. Results of a randomized, double-blind, placebo-controlled, phase III trial of trifluridine/tipiracil (TAS-102) monotherapy in Asian patients with previously treated metastatic colorectal cancer: the TERRA study. J Clin Oncol. 2018;36(4):350–358. doi: 10.1200/JCO.2017.74.3245. [DOI] [PubMed] [Google Scholar]
- 16.Yoshino T., Mizunuma N., Yamazaki K., et al. TAS-102 monotherapy for pretreated metastatic colorectal cancer: a double-blind, randomised, placebo-controlled phase 2 trial. Lancet Oncol. 2012;13(10):993–1001. doi: 10.1016/S1470-2045(12)70345-5. [DOI] [PubMed] [Google Scholar]
- 17.Van Cutsem E., Mayer R.J., Laurent S., et al. The subgroups of the phase III RECOURSE trial of trifluridine/tipiracil (TAS-102) versus placebo with best supportive care in patients with metastatic colorectal cancer. Eur J Cancer. 2018;90:63–72. doi: 10.1016/j.ejca.2017.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yoshino T., Shinozaki E., Yamazaki K., et al. PD-014 Final survival results and onset of neutropenia as an indicator of therapeutic effect in phase 2 of TAS-102 vs placebo with metastatic colorectal cancer (J003-10040030) Ann Oncol. 2016;27:ii107. [Google Scholar]
- 19.Katsahian S., Latouche A., Mary J.Y., Chevret S., Porcher R. Practical methodology of meta-analysis of individual patient data using a survival outcome. Contemp Clin Trials. 2008;29(2):220–230. doi: 10.1016/j.cct.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 20.Package ‘meta’. 2021 1 April 2021. General Package for Meta-Analysis. Version 4.18-0 https://cran.r-project.org/web/packages/meta/meta.pdf Available at.
- 21.Previous Releases of R for Windows. 1 April 2021. R 3.6.1 (July 2019) https://cran.r-project.org/bin/windows/base/old/ Available at.
- 22.Garcia-Carbonero N., Martinez-Useros J., Li W., et al. KRAS and BRAF mutations as prognostic and predictive biomarkers for standard chemotherapy response in metastatic colorectal cancer: a single institutional study. Cells. 2020;9(1):219. doi: 10.3390/cells9010219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Camaj P., Primo S., Wang Y., et al. KRAS exon 2 mutations influence activity of regorafenib in an SW48-based disease model of colorectal cancer. Future Oncol. 2015;11(13):1919–1929. doi: 10.2217/fon.15.97. [DOI] [PubMed] [Google Scholar]
- 24.Bai B., Shan L., Xie B., et al. Mutations in KRAS codon 12 predict poor survival in Chinese patients with metastatic colorectal cancer. Oncol Lett. 2018;15(3):3161–3166. doi: 10.3892/ol.2017.7709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen D., Wu Y.S., Lin H., Wang Y., Li L., Zhang T. Efficacy and safety of TAS-102 in refractory metastatic colorectal cancer: a meta-analysis. Cancer Manag Res. 2018;10:2915–2924. doi: 10.2147/CMAR.S174584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kwakman J.J.M., Vink G., Vestjens J.H., et al. Feasibility and effectiveness of trifluridine/tipiracil in metastatic colorectal cancer: real-life data from The Netherlands. Int J Clin Oncol. 2018;23(3):482–489. doi: 10.1007/s10147-017-1220-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tabernero J., Argiles G., Sobrero A.F., et al. Effect of trifluridine/tipiracil in patients treated in RECOURSE by prognostic factors at baseline: an exploratory analysis. ESMO Open. 2020;5(4) doi: 10.1136/esmoopen-2020-000752. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.