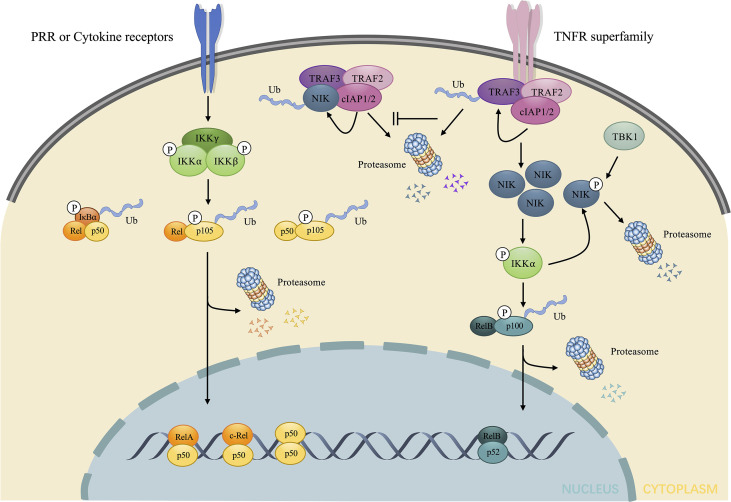
Figure 1.
Brief illustration of the activation and regulation of the two major NF-κB signaling pathway. The canonical pathway is induced downstream of the stimulation of LPS and cytokines. Such stimuli mediate the activation of IKK, consisting of three subunits: IKKα, IKKβ, and IKKγ, which in turn phosphorylates and elicits the ubiquitin dependent processing of IκB and p105, leading to the nuclear translocation of RelA/p50, c-Rel/p50, p50/p50 dimers and the regulation of target gene expression. The non-canonical pathway is induced downstream of TNFR superfamily. In the absence of stimuli, NIK undergoes ubiquitin-dependent degradation mediated by NIK ubiquitin ligase, composed of TRAF3, TRAF2 and cIAP1/2. Under stimuli, TRAF3 undergoes ubiquitin-dependent degradation, which allows the accumulation of NIK. NIK directly phosphorylates and activates IKKα, contributing to the phosphorylation and ubiquitin dependent processing of p100 to p52. The nuclear accumulation of p52/RelB dimers changes transcriptional activity of target genes. The excessive NIK can also be evacuated via negative feedback mechanisms. IKKα can phosphorylate NIK, resulting in the direct proteasome-mediated degradation without the involvement of NIK ubiquitin ligase. TBK1 downstream of CD40 and BAFF also phosphorylates NIK and induces degradation. LPS, lipopolysaccharides; IKK, IκB kinase; TNFR, tumor necrosis factor receptor; NIK, NF-κB inducing kinase; TBK1, TANK-binding kinase 1; BAFF, B-cell activating factor belonging to the TNF family.