Figure 1. Cryo-EM structure of 1D12 Fab in complex with H7 SH13 HA trimer at 2.8 Å resolution.
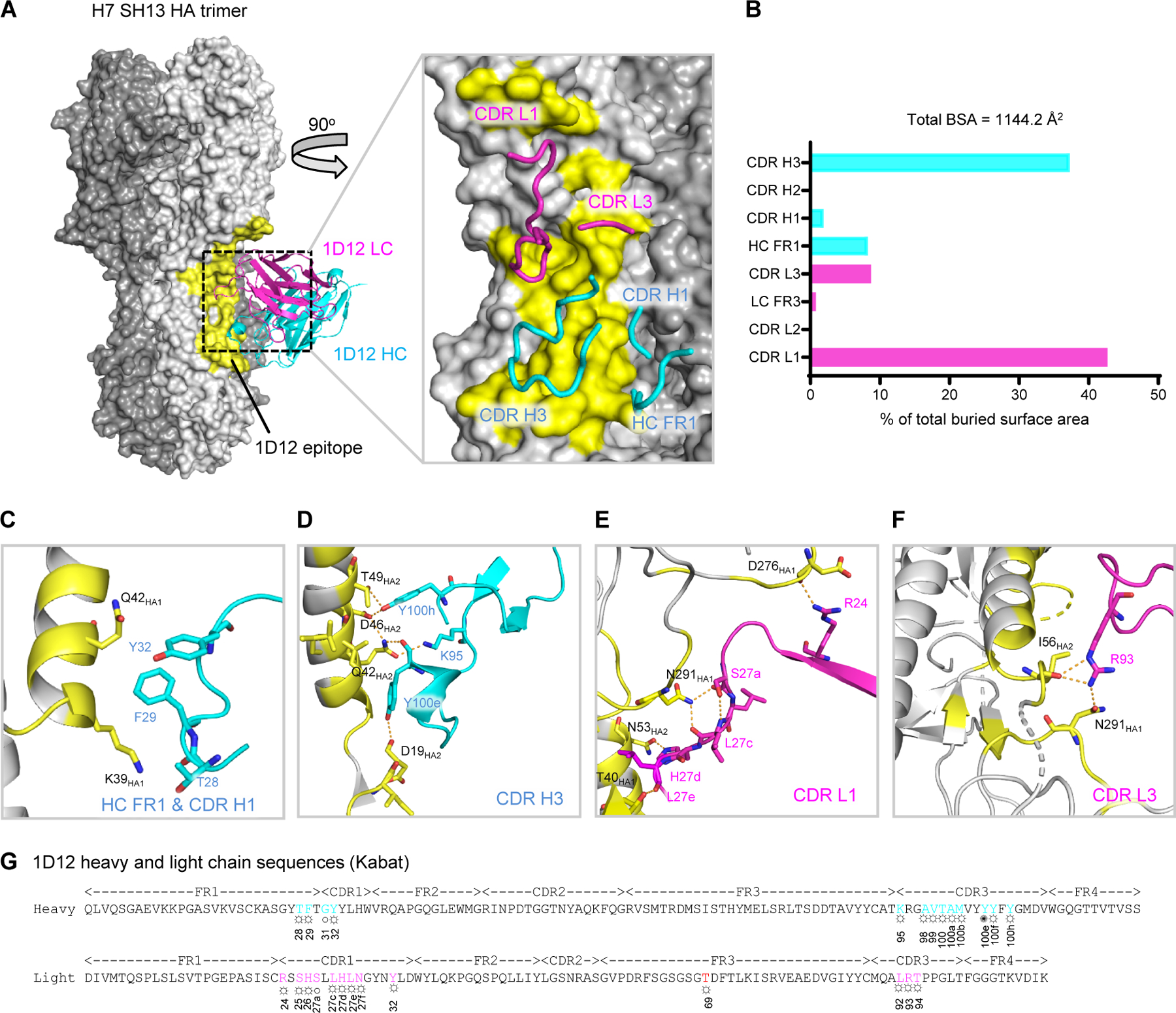
(A) Overall structure of 1D12 Fab bound to the H7 SH13 HA trimer. Only one antibody is shown for clarity. The HA trimer is shown in gray with varied shades for the protomers. The 1D12 epitope is colored yellow; heavy and light chain variable domains colored cyan and magenta, respectively. Zoom-in view highlights interactions of CDR and FR loops with HA. (B) Percentage of total buried surface area on SH13 HA contributed by the FRs and CDRs of 1D12 Fab. Buried surface areas were calculated with PISA (https://www.ebi.ac.uk/pdbe/pisa/). (C-F) CDR interactions show in ribbons with interacting residues in stick representation, colored as in (A). (G) 1D12 sequence annotated with Kabat numbering scheme. Heavy and light residues in contact with HA are colored cyan and magenta. ○ denotes main-chain interaction, ☼ denotes side-chain interaction and ✺ denotes both main- and side-chain interactions. See also Figures S1 and S2, and Tables S1 and S2.
