Figure 2. ARAF increases GTP-bound WT RAS in a kinase-independent manner. See also Figure S2.
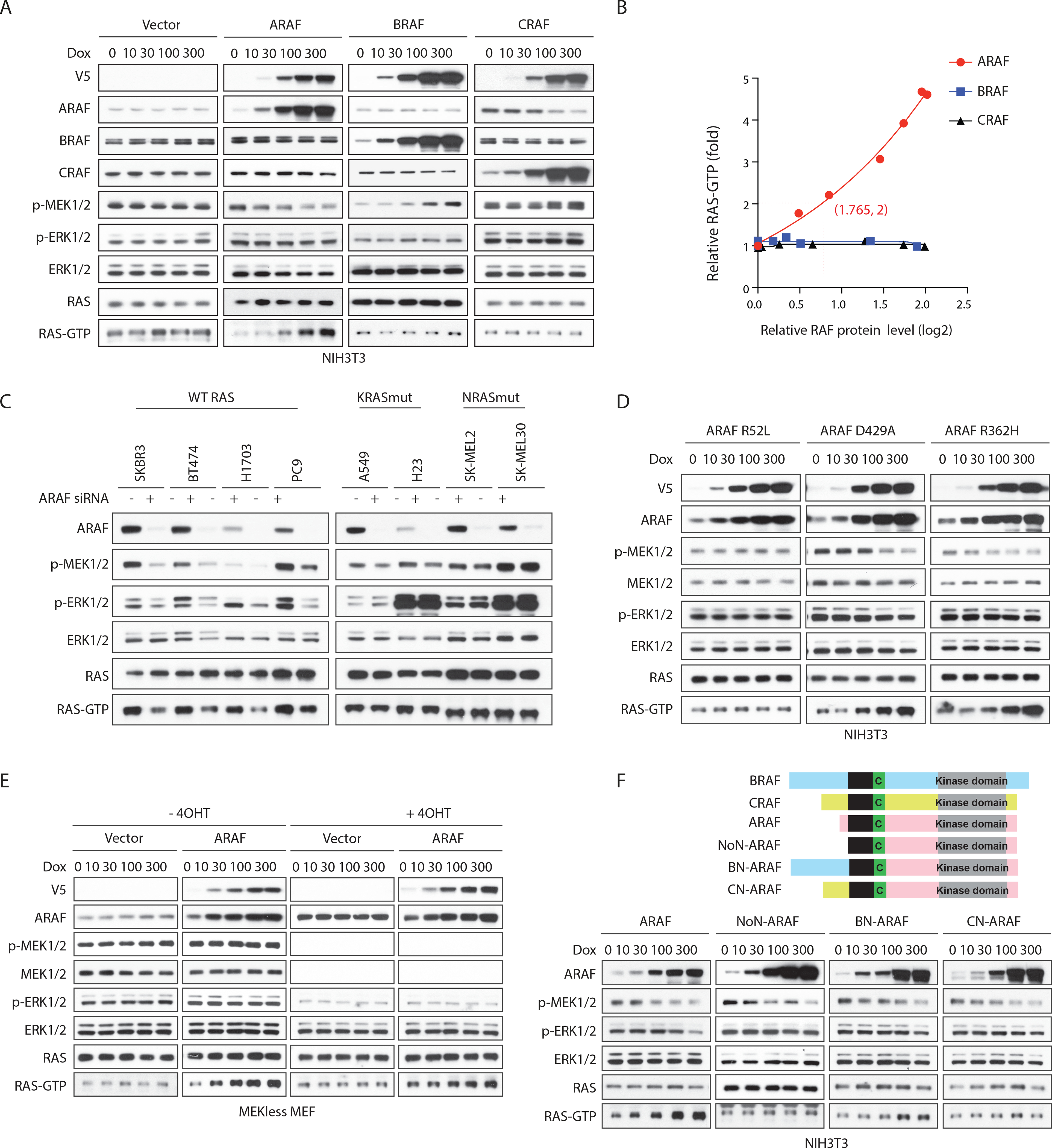
(A and B) NIH3T3 cells expressing doxycycline-inducible A-, B- or C-RAF were treated with doxycycline for 48 hours and subjected to western blot (A). RAS-GTP levels were quantified (B).
(C) SKBR3, BT474, H1703, PC9, A549, H23, SK-MEL-2 and SK-MEL30 cells were transfected with scramble or ARAF siRNA. Cells were collected 48 hours after transfection.
(D) NIH3T3 cells expressing doxycycline-inducible ARAF mutants were treated with doxycycline for 48 hours.
(E) MEK-less MEF cells expressing vector or doxycycline-inducible WT ARAF were grown in medium without or with Adeno-Cre particles plus 1 μM 4-OHT for a week to generate isogenic cells expressing or lacking MEK1. Cells were then treated with doxycycline for 48 hours.
(F) Upper panel, schematic representation of functional domains of RAF proteins and engineered ARAF fusion proteins with different N-terminus: NoN-ARAF, 18 amino acids of ARAF N-terminus is deleted; BN-ARAF, 18 amino acids of ARAF N-terminus is replaced by 154 amino acids from BRAF N-terminus; CN-ARAF, 18 amino acids of ARAF N-terminus is replaced by 55 amino acids from CRAF N-terminus. Lower panel, NIH3T3 cells expressing doxycycline-inducible WT or engineered ARAF variants were treated with doxycycline for 48 hours.
