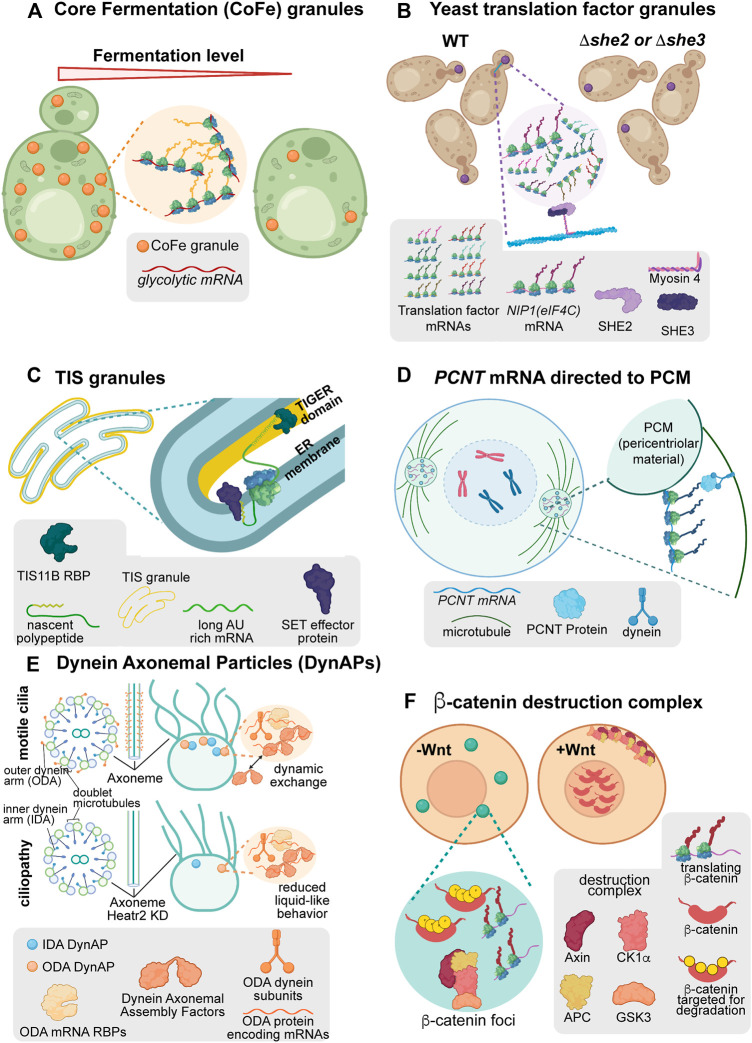
FIGURE 3.
Condensates are associated with diverse forms of post-transcriptional regulation. (A) Saccharomyces cerevisiae translation factor granules are polarized translation factory condensates associated with translation at regions of growth. When the SHE2 or SHE3 genes are removed from cells, these condensates no longer polarize in lab or wild isolate strains. (B) Core Fermentation (CoFe) granules in S. cerevisiae are translation factory condensates associated with the translation of glycolytic components under fermentative conditions. (C) TIS granules in human cell lines are mesh-like condensates interweaved with the ER. In these condensates, TIS11B associates with AU rich elements present in the long 3′UTR isoform of CD47 to facilitate interaction with its effector protein, SET, upon translation. This condensate-associated interaction promotes increased association of CD47 protein with the cell membrane. (D) In human cells, the PCNT RNA is actively transported towards the centromere while translating to facilitate rapid incorporation into transient pericentriolar condensates. These condensates support the organization of the pericentriolar material and microtubules to allow mitosis to occur normally. (E) Dynein axonemal particles (DynAPs) spatially organize dynein proteins within a condensate environment to promote their appropriate assembly in a “reaction crucible” mechanism. When the condensate environment is disrupted through depletion of Heatr2, Dynein complex assembly is disrupted, axonemal Dynein organization is perturbed, and cilial beating is decreased. (F) The ß-catenin destruction complex is a condensate which forms throughout the cell cycle to degrade the constitutively translated ß-catenin protein. Upon induction of Wnt signaling, the components of the destruction complex are modified causing the complex to disassemble and become sequestered at the plasma membrane. When the destruction complex is sequestered, nascent ß-catenin protein can translocate to the nucleus to perform its functions in Wnt signaling.