Abstract
Objectives
The peak expiratory flow rate (PEFR) is known to decrease in patients with sarcopenia. However, little is known about the clinical impact of the PEFR in idiopathic pulmonary fibrosis (IPF). This study aimed to confirm whether a decrease in PEFR over 6 months was associated with survival in IPF patients.
Methods
Consecutive IPF patients who had been assessed at a single center were retrospectively analyzed. The relative decline in PEFR over 6 months was assessed. Survival analyses were performed by univariate and multivariate Cox proportional hazard models.
Results
A total of 61 eligible cases (average age 70 years) were examined, and 21 patients (34.4%) died. The univariate Cox regression analysis showed that the body mass index, baseline % predicted forced vital capacity (FVC), baseline % predicted PEFR, % predicted diffusion capacity for carbon monoxide (DLCO), relative decline in FVC, and relative decline in PEFR were prognostic factors. On multivariate analyses, relative decline in PEFR (hazard ratio [HR] 1.037, p < .05) and baseline % predicted FVC (HR 0.932, p < .001) were independent prognostic factors, whereas relative decline in FVC was not.
Conclusion
A decrease in PEFR after 6 months may predict worse survival in patients with IPF.
Keywords: Peak expiratory flow rate, idiopathic pulmonary fibrosis, mortality, erector spinae muscle, sarcopenia
Introduction
Idiopathic pulmonary fibrosis (IPF) is a fibrotic and progressive pulmonary disease that leads to death in most patients. 1 The median survival times of IPF patients were poor at 3 years before the development of anti-fibrotic drugs.1–3 This disease is characterized by decreased lung volumes and reduced gas exchange, and it is associated with symptoms of progressive dyspnea, cough, and reduced exercise capacity. Several known prognostic factors for IPF are: dyspnea score, 4 pulmonary function,5–7 disease severity,2,8 functional exercise capacity,9,10 and fibrotic changes on high-resolution computed tomography (HRCT). 11 Of pulmonary function variables, a decline in forced vital capacity (FVC) is widely known as a useful prognostic factor.5–7
Sarcopenia is a syndrome characterized by a progressive and generalized loss of systemic skeletal muscle mass and strength, and it carries the risk of poor outcomes such as physical disability, poor quality of life, and death. 12 It has been reported that the loss of skeletal muscle mass assessed using computed tomography (CT) images is associated with a poor prognosis in patients with IPF.13,14 Furthermore, we have previously reported that the decrease over the course of 6 months of the cross-sectional area (CSA) of the erector spinae muscle (ESMCSA), evaluated using CT images, was a strong predictor of mortality in patients with IPF. 15 In that study, baseline ESMCSA was not a prognostic factor on multivariate analysis.
The peak expiratory flow rate (PEFR) is obtained by routine spirometry. PEFR is the maximum flow rate generated during a forceful exhalation, starting from full lung inflation. PEFR well reflects large airway flow in patients with asthma and chronic obstructive pulmonary disease, but in patients with IPF, it would strongly depend on the respiratory muscular strength of the patient. Generally, in patients with IPF, radial traction due to scarring of the surrounding parenchyma prevents dynamic compression of the airway and, subsequently, does not reduce PEFR. 16
In previous studies, a decrease in PEFR was correlated with sarcopenia and skeletal muscle mass loss.17,18 Therefore, we hypothesized that a decrease in PEFR over the course of 6 months would be associated with poor survival in patients with IPF.
Methods
This study protocol was approved by the Ethics Committee of Nagoya City University Hospital (approval number 60-21-055) and carried out in accordance with approved guidelines. The need for patient approval and/or informed consent was waived by the Ethics Committee of Nagoya City University Hospital due to the retrospective nature of the study.
Patients
The clinical records of 80 consecutive patients with IPF who were referred to the Nagoya City University Hospital between October 2013 and April 2020 were retrospectively reviewed. IPF was diagnosed through multidisciplinary discussions according to international guidelines.1,19,20 The exclusion criteria were as follows: patients who died sooner than 6 months from baseline; patients whose pulmonary function was not assessed at the initial visit or after 6 months due to any cause; and patients who had lung cancer at baseline. The observation period commenced from the time of the patient’s initial evaluation until death, or 30 April 2021, whichever was sooner. Censored cases were evaluated to confirm their life or death status by telephone, as often as was possible. A pulmonary rehabilitation program was performed during hospitalization by patients who had acute exacerbations of IPF or pneumonia. Outpatients did not perform rehabilitation.
Pulmonary function tests
All patients completed pulmonary function tests using spirometry (CHESTAC-8900; Chest, Tokyo, Japan) according to the ATS/ERS criteria. 21 The diffusion capacity for carbon monoxide (DLco) was also measured (CHESTAC-8900). Percentage of predicted FVC (%FVC), percentage of predicted forced expiratory volume in 1.0 s (FEV1), and percentage of predicted DLco (%DLco) were calculated based on the patients’ height, age, and sex, according to the Japanese standardized methods. 22 Percent (%) predicted PEFR was calculated based on the prediction equation reported by Nunn et al. 23 The percent (%) predicted maximum mid-expiratory flow rate (MMF) was calculated using the prediction equation reported by Schmidt et al. 24
Derivation of ESMCSA by imaging analysis software
SYNAPSE VINCENT (Fujifilm Medical Systems, Tokyo, Japan) CT imaging analysis software was used for the derivation of ESMCSA. ESMCSA was calculated manually according to a previously published method.15,25 Briefly, ESMCSA was measured on a single-slice axial CT image at the level of the spinous process of the 12th thoracic vertebra. For the quantitative analysis of the ESMs, chest HRCT images were reconstructed using the mediastinal window setting (window level, 40 HU; window width, 300 HU). The left and right ESMs were identified and manually shaded, and the ESM area was reported as the sum of the right and left ESMs.
The Charlson comorbidity index
The Charlson comorbidity index was calculated according to a previously reported method. 26
Statistical analyses
Continuous variables are presented as means (±standard deviations) or medians [interquartile ranges]. Categorical variables are presented as numbers and percentages. The relationships between continuous variables were evaluated using Spearman’s rank correlation coefficients. Univariate Cox proportional hazard models were used to examine the associations of selected variables with survival. Stepwise multivariate Cox proportional regression analysis was performed including the significant variables on univariate analysis, with subsequent forward stepwise selection. To avoid multicollinearity, some of the highly correlated variables were excluded on multivariate analysis if they had Spearman’s correlation coefficients greater than 0.7. Survival times were estimated using the Kaplan–Meier method and compared using the log-rank test. To determine the optimal cutoff value for predicting 2-years mortality, receiver operating characteristic (ROC) curves were constructed. Multiple linear regression analysis was performed to identify clinical parameters related to relative decline in PEFR. To assess multicollinearity, variance inflation factor (VIF) values were computed. The differences between patients separated by cutoff values of the relative decline in PEFR were analyzed using Student’s t-test or the Mann-Whitney U test for continuous variables and the Chi-squared test or Fisher’s exact test for categorical variables, as appropriate. p values < .05 were considered significant. Statistical analyses were conducted using IBM SPSS Statistics version 28 (IBM Corp., Armonk, NY, USA).
Results
Patients’ characteristics
Thirteen patients whose pulmonary function had not been assessed at the initial visit or after 6 months were excluded. Among them, one patient died before 6 months from baseline. Six patients who had lung cancer at baseline were excluded. Thus, the study population consisted of 61 patients with IPF. The median time from initial assessment to spirometry 6 months later was 175 [158–210] days. The patients’ characteristics at baseline and after 6 months are shown in Table 1. The median baseline PEFR was 7.19 [5.50–8.42] L/s, whereas that after 6 months was 6.68 [5.30–8.52] L/s.
Table 1.
Patients’ characteristics.
| Variable | 0 Months | 6 Months |
|---|---|---|
| Total, n | 61 | 61 |
| Age, y | 70.1 ± 7.9 | |
| Sex, female, n (%) | 7 (11.5%) | |
| Never smoker, n (%) | 12 (19.7%) | |
| Ex-smoker, n (%) | 44 (72.1%) | |
| Current smoker, n (%) | 5 (8.2%) | |
| Smoking history, pack-years | 38.0 (15.0–56.0) | |
| Body mass index, kg/m2 | 23.6 ± 3.3 | |
| Histological diagnosis, n (%) | 9 (14.8%) | |
| FVC, L | 2.73 (1.87–3.26) | 2.68 (1.85–3.25) |
| FVC, % predicted | 83.7 (69.4–92.5) | 81.6 (69.0–93.2) |
| PEFR, L/s | 7.19 (5.50–8.42) | 6.68 (5.30–8.52) |
| PEFR, % predicted | 81.2 (70.2–92.6) | 77.0 (64.5–94.3) |
| FEV1, L | 2.18 (1.66–2.60) | 2.13 (1.53–2.59) |
| FEV1, % predicted | 82.2 (74.5–91.7) | 81.6 (68.3–92.2) |
| FEV1/FVC, % | 83.0 (77.1–87.6) | 81.6 (76.1–86.4) |
| MMF, L | 2.29 (1.59–3.05) | 2.15 (1.52–2.96) |
| MMF, % predicted | 82.5 (57.8–103.1) | 77.4 (53.2–97.9) |
| DLCO, % predicted a | 73.0 (54.8–85.8) | |
| ESMCSA, cm2b | 29.1 ± 6.4 |
Data are presented as means (± standard deviation), medians [interquartile range], or numbers (%).
FVC: forced vital capacity; PEFR: peak expiratory flow rate; FEV1: forced expiratory volume in 1.0 s; MMF: maximum mid-expiratory flow rate; DLCO: diffusion capacity of the lung for carbon monoxide; ESMCSA; the cross-sectional area of the erector spinae muscle evaluated using computed tomography images.
aTwo patients could not perform the DLCO maneuver.
bNine cases could not be evaluated.
Correlation between PEFR and ESMCSA
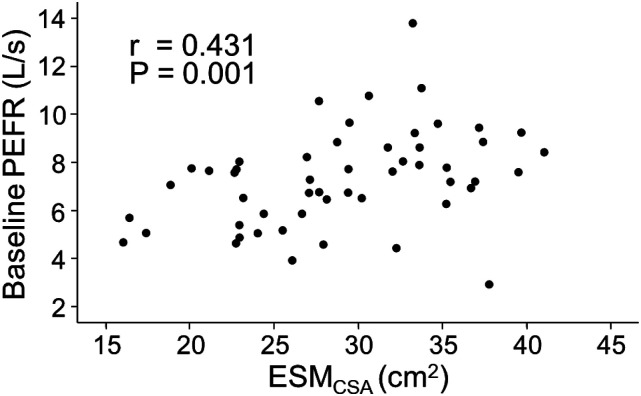
It has been reported that PEFR is an indicator of sarcopenia. 17 On the other hand, assessment of the ESMCSA from chest CT images has been used to evaluate sarcopenia in patients with chronic lung disease.13,25 Therefore, the relationship between PEFR and ESMCSA was examined in patients with IPF. The mean ESMCSA was 29.1 ± 6.4 cm2 in the present cohort. Nine patients' ESMCSA values could not be analyzed because their CT lacked the level of the spinous process of the 12th thoracic vertebra. Baseline PEFR was significantly correlated with ESMCSA (r = 0.431, p < .005) (Figure 1).
Figure 1.
Correlation between PEFR and ESMCSA. The correlation between baseline PEFR and ESMCSA is shown. PEFR: peak expiratory flow rate; ESMCSA: the cross-sectional area of the erector spinae muscle evaluated on computed tomography images.
Relative decline in PEFR and other respiratory function parameters
The median relative decline in PEFR was 2.90 (−7.27–11.54) %. In addition, the median relative decline in FVC was 2.45 (−2.49–5.54) %, the median relative decline in FEV1 was 3.19 (−2.40–9.03) %, and the median relative decline in MMF was 4.44 (−12.81–15.74) %.
Prognostic survey
Twenty-one of 61 patients (34.4%) died during the study period. The follow-up time of the 61 patients was 1017 days (530–1623). Four patients were lost to follow-up. The median survival estimate of the cohort was 2214 days.
Prognostic factors on univariate and multivariate Cox regression analyses
Hazard ratios (HRs) and 95% CIs of this cohort on univariate and multivariate Cox regression analyses are shown in Table 2. The univariate Cox regression analysis showed that body mass index (HR 0.859, 95% CI 0.742–0.995, p < .05), baseline %FVC (HR 0.941, 95% CI 0.917–0.966, p < .001), baseline %PEFR (HR 0.978, 95% CI 0.956–0.999, p < .05), baseline %FEV1 (HR 0.947, 95% CI 0.919–0.977, p < .001), baseline %DLco (HR 0.973, 95% CI 0.952–0.995, p < .05), relative decline in FVC (HR 1.035, 95% CI 1.001–1.070, p < .05), relative decline in PEFR (HR 1.035, 95% CI 1.002–1.069, p < .05), and relative decline in FEV1 (HR 1.041, 95% CI 1.008–1.075, p < .05) were prognostic factors. %FEV1 was excluded from multivariate analysis because of its high correlation with %FVC (Spearman’s correlation coefficient, r = 0.797, p < .001). Furthermore, relative decline in FEV1 was excluded from multivariate analysis because of its high correlation with relative decline in FVC (r = 0.731, p < .001). On multivariate analyses, relative decline in PEFR (HR 1.037, 95% CI 1.002–1.072, p < .05) and baseline % predicted FVC (HR 0.932, 95% CI 0.905–0.960, p < .001) were independent prognostic factors, whereas relative decline in FVC and baseline %PEFR were not.
Table 2.
Prediction of mortality by univariate and multivariate Cox proportional hazards analyses.
| Predictor | HR | 95% CI | p-value |
|---|---|---|---|
| Univariate analysis | |||
| Age | 1.067 | 0.991–1.149 | .085 |
| Sex, female | 0.780 | 0.182–3.356 | .739 |
| Body mass index | 0.859 | 0.742–0.995 | <.05 |
| Baseline FVC, % predicted | 0.941 | 0.917–0.966 | <.001 |
| Baseline PEFR, % predicted | 0.978 | 0.956–0.999 | <.05 |
| Baseline FEV1, % predicted | 0.947 | 0.919–0.977 | <.001 |
| Baseline MMF, % predicted | 1.008 | 0.995–1.020 | .248 |
| Baseline DLco, % predicted | 0.973 | 0.952–0.995 | <.05 |
| Baseline ESMCSA, cm2 | 1.000 | 0.999–1.000 | .314 |
| Relative decline in FVC, % | 1.035 | 1.001–1.070 | <.05 |
| Relative decline in PEFR, % | 1.035 | 1.002–1.069 | <.05 |
| Relative decline in FEV1, % | 1.041 | 1.008–1.075 | <.05 |
| Relative decline in MMF, % | 0.990 | 0.976–1.005 | .205 |
| Multivariate analysis (Stepwise) | |||
| Baseline FVC, % predicted | 0.932 | 0.905–0.960 | <.001 |
| Relative decline in PEFR, % | 1.037 | 1.002–1.072 | <.05 |
HR: hazard ratio; CI: confidence interval; FVC: forced vital capacity; PEFR: peak expiratory flow rate; FEV1: forced expiratory volume in 1.0 s; MMF: maximum mid-expiratory flow rate; DLCO: diffusion capacity of the lung for carbon monoxide; ESMCSA: the cross-sectional area of the erector spinae muscle evaluated using computed tomography images.
HRs and 95% CIs for each parameter on univariate and multivariate Cox regression analyses of only baseline data are shown in Supplemental Table S1.
Kaplan–Meier curves and a log-rank test
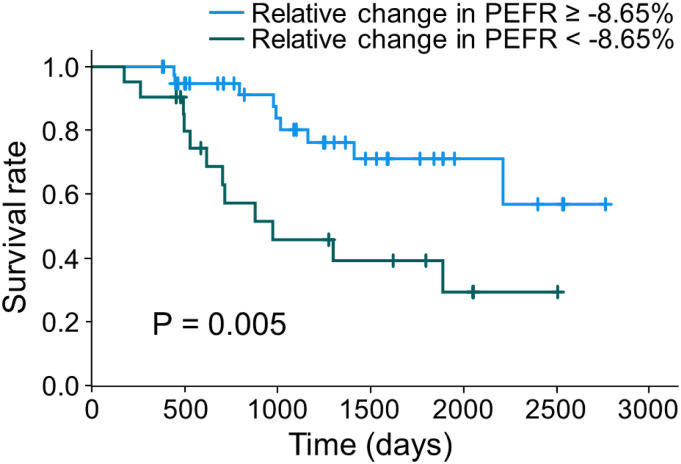
The Kaplan–Meier curves of this cohort are shown in Figure 2. To determine the optimal cutoff value for predicting 2-years mortality, an ROC analysis was performed by using the data of 47 patients. A cutoff value of 8.65% (area under the curve = 0.737, specificity: 0.778, sensitivity: 0.763) was identified. The mean survival estimates were as follows: relative decline in PEFR ≥8.65%, 1335 days, and relative decline in PEFR <8.65%, 2169 days. Relative decline in PEFR ≥8.65% had a significantly worse prognosis (p < .01, log-rank test).
Figure 2.
Kaplan–Meier curves and a log-rank test. Kaplan–Meier survival curves stratified by relative decline in PEFR. The cutoff value was set at 8.65%. Patients with a relative decline in PEFR over 8.65% had significantly worse survival (p < .01, log-rank test). PEFR: peak expiratory flow rate.
Correlations between the relative decline in PEFR and other clinical parameters
The correlations between the relative decline in PEFR and other clinical parameters are shown in Table 3. Baseline %FVC was slightly correlated. On the other hand, relative decline in FVC was not correlated with relative decline in PEFR. Supplemental Figure S1 shows the correlations of relative decline in PEFR with relative decline in FVC (S1A) and baseline %FVC (S1B).
Table 3.
Correlations between relative decline in PEFR and clinical parameters.
| Variable | r | 95% CI | p-value |
|---|---|---|---|
| Age | 0.151 | −0.112–0.395 | .244 |
| Body mass index | 0.128 | −0.135–0.375 | .324 |
| Baseline FVC, % predicted | −0.270 | −0.494–0.012 | <.05 |
| Baseline PEFR, % predicted | 0.106 | −0.157–0.355 | .415 |
| Baseline FEV1, % predicted | −0.235 | −0.465–0.026 | .069 |
| Baseline MMF, % predicted | −0.013 | −0.271–0.247 | .919 |
| Baseline DLCO, % predicted | −0.115 | −0.367–0.153 | .388 |
| Baseline ESMCSA | 0.065 | −0.220–0.339 | .648 |
| Relative decline in FVC, % | 0.080 | −0.183–0.332 | .542 |
| Relative decline in FEV1, % | 0.139 | −0.125–0.384 | .287 |
| Relative decline in MMF, % | 0.067 | −0.195–0.321 | .607 |
CI: confidence interval; FVC: forced vital capacity; PEFR: peak expiratory flow rate; FEV1: forced expiratory volume in 1.0 s; MMF: maximum mid-expiratory flow rate; DLCO: diffusion capacity of the lung for carbon monoxide; ESMCSA: the cross-sectional area of the erector spinae muscle evaluated using CT images.
Multiple linear regression analysis for decline in PEFR
Multiple linear regression analysis was performed to identify clinical parameters related to relative decline in PEFR (Table 4). Age, baseline %FVC, and baseline %PEFR were independent factors contributing to relative decline in PEFR. The VIF values of age, body mass index, %FVC, %PEFR, %MMF, %DLco were 1.17, 1.13, 1.72, 1.39, 1.15, and 1.46, respectively. All VIF values were <10, and there was no multicollinearity among these factors.
Table 4.
Multiple linear regression analysis for decline in PEFR.
| B | 95% CI | Standardized β | p-value | VIF | |
|---|---|---|---|---|---|
| Age | 0.60 | 0.03–1.17 | 0.26 | <.05 | 1.17 |
| Body mass index | 0.76 | −0.56–2.08 | 0.14 | .25 | 1.13 |
| Baseline FVC, % predicted | −0.60 | −0.94–−0.26 | −0.53 | <.001 | 1.72 |
| Baseline PEFR, % predicted | 0.49 | 0.24–0.75 | 0.53 | <.001 | 1.39 |
| Baseline MMF, % predicted | −0.03 | −0.15–0.09 | −0.07 | .58 | 1.15 |
| Baseline DLco, % predicted | 0.03 | −0.21– 0.27 | 0.04 | .79 | 1.46 |
CI: confidence interval; VIF: variance inflation factor values; FVC: forced vital capacity; PEFR: peak expiratory flow rate; MMF: maximum mid-expiratory flow rate; DLCO: diffusion capacity of the lung for carbon monoxide.
Characteristics of IPF patients separated by the cutoff value
The clinical characteristics of the IPF patients classified by the cutoff value of 8.65% for relative decline in PEFR are shown in Supplemental Table S2. There was a significant difference in baseline %FVC (p < .05) and baseline %FEV1 (p < .05) between the two groups. In addition, the clinical characteristics with and without anti-fibrotic drug use are shown in Supplemental Table S3.
Discussion
Several studies have reported that a decrease in PEFR was an indicator of sarcopenia and skeletal muscle mass loss in older adults.17,18 Kera et al. 27 created a definition of respiratory sarcopenia based on the PEFR (cut-off values were 4.40 L/s for men, 3.21 L/s for women) and demonstrated that respiratory sarcopenia correlated with conventional sarcopenia. Evaluation of ESMCSA on chest CT images has also been used to assess sarcopenia in patients with chronic lung disease.13,25 Regarding the baseline ESMCSA, Suzuki et al. reported that baseline ESMCSA was related to prognosis, 13 whereas Nakano et al. 15 reported that it was not related to prognosis. In the present study, baseline % predicted PEFR was one of the prognostic factors on univariate Cox regression analysis, whereas baseline ESMCSA was not. Baseline PEFR was correlated with ESMCSA in patients with IPF (Figure 1). The measurement of PEFR seems to be simpler than the analysis of ESMCSA, and we considered it as an indicator of sarcopenia that predicts prognosis in patients with IPF.
The present study demonstrated that a decrease in PEFR over 6 months was a strong prognostic factor in IPF patients. PEFR can be obtained by routine spirometry. However, to the best of our knowledge, this is the first study to examine the use of PEFR for mortality analysis in patients with IPF. Interestingly, on multivariate analysis, relative decline in PEFR (HR 1.037, 95% CI 1.002–1.072, p < .05) was an independent prognostic factor, whereas the relative decline in FVC was not.
Two anti-fibrotic therapies, nintedanib and pirfenidone, have been approved for the treatment of IPF. Anti-fibrotic drugs have been shown to significantly reduce the annual rate of decrease in FVC, and they appear to reduce the risk of all-cause mortality. 28 In the present study, more than 80% of patients were treated with anti-fibrotic drugs. This may cause a reduction in the relative decline in FVC. Even if the effects of anti-fibrotic drugs are taken into account, the decrease in PEFR may have a strong impact on the survival rate in patients with IPF.
Regarding the loss of skeletal muscles in patients with IPF, a low ESMCSA on CT images13–15 and a low fat-free mass index by bioelectrical impedance analysis 29 were demonstrated to be associated with a poor prognosis. In patients with IPF, progressive loss of systemic skeletal muscles can be thought to decrease daily activity, worsen chronic respiratory failure, and increase infectious pneumonia that leads to death. 30
Multiple linear regression analysis showed that age, baseline %FVC, and baseline %PEFR were independent factors contributing to relative decline in PEFR. A decrease in PEFR can be caused by progressive weakness of the respiratory muscles.17,18,27 Loss of respiratory muscle mass may be induced by systemic inflammation, malnutrition, enhanced energy expenditure, and aging. More advanced and elderly IPF patients might have greater losses of systemic skeletal muscle mass and strength. It is possible that a rehabilitation program and nutritional guidance for outpatients with IPF could improve the PEFR decline.
In the present study, baseline % predicted PEFR was also a prognostic factor (HR 0.978, 95% CI 0.956–0.999, p < .05) on univariate analysis, but not on multivariate analysis. The relative decline in PEFR over 6 months was considered to be more useful in predicting survival than the baseline % predicted PEFR.
Recently, 3-month change in FVC has shown prognostic potential in a large IPF meta-analysis. 31 In the present study, the reason for choosing a 6-month period was that most patients had respiratory function tests performed every 6 months. Most patients visited our hospital every 2 months or every 3 months. It would be useful if short-term changes of markers were found to be prognostic factors. Investigation of daily home spirometry 32 might provide more useful markers.
The present study has the following limitations. First, the results were obtained by a retrospective analysis of all Japanese patients from a single center with a small sample size. Second, DLco and ESMCSA after 6 months were not analyzed because there were many cases of missing data. Third, there may be survivor bias and significant lead-time bias. Fourth, an ROC analysis was performed using data of only 47 patients (77% of the cohort) to determine the optimal cutoff values for predicting 2-years mortality. Future studies are necessary to examine the cutoff values in more facilities and in larger samples. The optimal cutoff value may be useful for physicians to note the possibility of weakened respiratory muscles, and to decide starting muscle training with rehabilitation and nutritional guidance. Thus, the present study can be considered a preliminary report. Further studies are warranted to elucidate the importance of relative decline of PEFR in patients with IPF.
In conclusion, a decrease in PEFR after 6 months could be a novel and useful prognostic factor in patients with IPF. Progression of respiratory sarcopenia can be considered the cause of the decrease in PEFR. It is important to pay attention to PEFR in the clinical practice of IPF.
Supplemental Material
Supplemental Material for Decreased peak expiratory flow rate associated with mortality in idiopathic pulmonary fibrosis: A preliminary report by Kohei Fujita, Hirotsugu Ohkubo, Akiko Nakano, Norihisa Takeda, Kensuke Fukumitsu, Satoshi Fukuda, Yoshihiro Kanemitsu, Takehiro Uemura, Tomoko Tajiri, Ken Maeno, Yutaka Ito, Tetsuya Oguri, Yoshiyuki Ozawa, Takayuki Murase and Akio Niimi in Chronic Respiratory Disease
Acknowledgements
The authors would like to thank Forte Science Communications (fortescience.com) for English language editing.
Author contributions: KoF and HO contributed equally to this study. KoF and HO drafted the submitted article and take responsibility for the integrity of the data and the accuracy of the data analysis. ANa, NT, KeF, SF, YK, TU, TT, KM, YI, TO, and ANi contributed to the interpretation of the manuscript. YO contributed as a radiologist. TM contributed as a pathologist.
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Supplemental Material: Supplemental Material for this article is available online.
ORCID iDs
Hirotsugu Ohkubo https://orcid.org/0000-0002-8538-1150
Ken Maeno https://orcid.org/0000-0002-4561-4044
References
- 1.Raghu G, Collard HR, Egan JJ, et al. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med 2011; 183: 788–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ley B, Ryerson CJ, Vittinghoff E, et al. A multidimensional index and staging system for idiopathic pulmonary fibrosis. Ann Intern Med 2012; 156: 684–691. [DOI] [PubMed] [Google Scholar]
- 3.Natsuizaka M, Chiba H, Kuronuma K, et al. Epidemiologic survey of Japanese patients with idiopathic pulmonary fibrosis and investigation of ethnic differences. Am J Respir Crit Care Med 2014; 190: 773–779. [DOI] [PubMed] [Google Scholar]
- 4.Nishiyama O, Taniguchi H, Kondoh Y, et al. A simple assessment of dyspnoea as a prognostic indicator in idiopathic pulmonary fibrosis. Eur Respir J 2010; 36: 1067–1072. [DOI] [PubMed] [Google Scholar]
- 5.Zappala CJ, Latsi PI, Nicholson AG, et al. Marginal decline in forced vital capacity is associated with a poor outcome in idiopathic pulmonary fibrosis. Eur Respir J 2010; 35: 830–836. [DOI] [PubMed] [Google Scholar]
- 6.Sharp C, Adamali HI, Millar AB. A comparison of published multidimensional indices to predict outcome in idiopathic pulmonary fibrosis. ERJ Open Res 2017; 3: 00096–02016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Paterniti MO, Bi Y, Rekić D, et al. Acute exacerbation and decline in forced vital capacity are associated with increased mortality in idiopathic pulmonary fibrosis. Ann Am Thorac Soc 2017; 9: 1395–1402. [DOI] [PubMed] [Google Scholar]
- 8.Jo HE, Glaspole I, Grainge C, et al. Baseline characteristics of idiopathic pulmonary fibrosis: analysis from the Australian Idiopathic Pulmonary Fibrosis Registry. Eur Respir J 2017; 49: 1601592. [DOI] [PubMed] [Google Scholar]
- 9.du Bois RM, Albera C, Bradford WZ, et al. 6-minute walk distance is an independent predictor of mortality in patients with idiopathic pulmonary fibrosis. Eur Respir J 2014; 43: 1421–1429. [DOI] [PubMed] [Google Scholar]
- 10.Vainshelboim B, Oliveira J, Fox BD, et al. The prognostic role of ventilatory inefficiency and exercise capacity in idiopathic pulmonary fibrosis. Respir Care 2016; 61: 1100–1109. [DOI] [PubMed] [Google Scholar]
- 11.Sumikawa H, Johkoh T, Colby TV, et al. Computed tomography findings in pathological usual interstitial pneumonia: relationship to survival. Am J Respir Crit Care Med 2008; 177: 433–439. [DOI] [PubMed] [Google Scholar]
- 12.Cruz-Jentoft AJ, Baeyens JP, Bauer JM, et al. Sarcopenia: European consensus on definition and diagnosis: report of the European working group on sarcopenia in older people. Age Aging 2010; 39: 412–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Suzuki Y, Yoshimura K, Enomoto Y, et al. Distinct profile and prognostic impact of body composition changes in idiopathic pulmonary fibrosis and idiopathic pleuroparenchymal fibroelastosis. Sci Rep 2018; 8: 14074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moon SW, Choi JS, Lee SH, et al. Thoracic skeletal muscle quantification: low muscle mass is related with worse prognosis in idiopathic pulmonary fibrosis patients. Respir Res 2019; 20: 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nakano A, Ohkubo H, Taniguchi H, et al. Early decrease in erector spinae muscle area and future risk of mortality in idiopathic pulmonary fibrosis. Sci Rep 2020; 10: 2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.West JB. Pulmonary physiology and pathophysiology: an integrated, case-based approach. 2nd ed. Philadelphia, PA: Lippincott Williams & Wilkins, 2007. [Google Scholar]
- 17.Kera T, Kawai H, Hirano H, et al. Relationships among peak expiratory flow rate, body composition, physical function, and sarcopenia in community-dwelling older adults. Aging Clin Exp Res 2018; 30: 331–340. [DOI] [PubMed] [Google Scholar]
- 18.Ohara DG, Pegorari MS, Oliveira dos Santos NL, et al. Respiratory muscle strength as a discriminator of sarcopenia in community-dwelling elderly: a cross-sectional study. J Nutr Health Aging 2018; 22: 952–958. [DOI] [PubMed] [Google Scholar]
- 19.Raghu G, Rochwerg B, Zhang Y, et al. An official ATS/ERS/JRS/ALAT clinical practice guideline: treatment of idiopathic pulmonary fibrosis. An update of the 2011 clinical practice guideline. Am J Respir Crit Care Med 2015; 192: e3–19. [DOI] [PubMed] [Google Scholar]
- 20.Raghu G, Remy-Jardin M, Myers JL, et al. Diagnosis of idiopathic pulmonary fibrosis. An Official ATS/ERS/JRS/ALAT clinical practice guideline. Am J Respir Crit Care Med 2018; 198: e44–68. [DOI] [PubMed] [Google Scholar]
- 21.Laszlo G. Standardisation of lung function testing: helpful guidance from the ATS/ERS Task Force. Thorax 2006; 61: 744–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kubota M, Kobayashi H, Quanjer PH, et al. Reference values for spirometry, including vital capacity, in Japanese adults calculated with the LMS method and compared with previous values. Respir Investig 2014; 52: 242–250. [DOI] [PubMed] [Google Scholar]
- 23.Nunn AJ, Gregg I. New regression equations for predicting peak expiratory flow in adults. Br Med J 1989; 298: 1068–1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schmidt CD, Dickman ML, Gardner RM, et al. Spirometric standards for healthy elderly man and women. Am Rev Resp Dis 1973; 108: 933–939. [DOI] [PubMed] [Google Scholar]
- 25.Tanimura K, Sato S, Fuseya Y, et al. Quantitative assessment of erector spinae muscles in patients with chronic obstructive pulmonary disease. Novel chest computed tomography-derived index for prognosis. Ann Am Thorac Soc 2016; 13: 334–341. [DOI] [PubMed] [Google Scholar]
- 26.Quan H, Li B, Couris CM, et al. Updating and validating the Charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am J Epidemiol 2011; 173: 676–682. [DOI] [PubMed] [Google Scholar]
- 27.Kera T, Kawai H, Hirano H, et al. Definition of respiratory sarcopenia with peak expiratory flow rate. J Am Med Dir Assoc 2019; 20: 1021–1025. [DOI] [PubMed] [Google Scholar]
- 28.Petnak T, Lertjitbanjong P, Thongprayoon C, et al. Impact of antifibrotic therapy on mortality and acute exacerbation in idiopathic pulmonary fibrosis: a systematic review and meta-analysis. Chest 2021; 160: 1751–1763. [DOI] [PubMed] [Google Scholar]
- 29.Nishiyama O, Yamazaki R, Sano H, et al. Fat-free mass index predicts survival in patients with idiopathic pulmonary fibrosis. Respirology 2017; 22: 480–485. [DOI] [PubMed] [Google Scholar]
- 30.Suzuki Y, Aono Y, Kono M, et al. Cause of mortality and sarcopenia in patients with idiopathic pulmonary fibrosis receiving antifibrotic therapy. Respirology 2021; 26: 171–179. [DOI] [PubMed] [Google Scholar]
- 31.Khan FA, Stewart I, Moss S, et al. Three-month FVC change: A trial endpoint for idiopathic pulmonary fibrosis based on individual participant data meta-analysis. Am J Respir Crit Care Med 2022; 205: 936–948. [DOI] [PubMed] [Google Scholar]
- 32.Russell AM, Adamali H, Molyneaux PL, et al. Daily home spirometry: an effective tool for detecting progression in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 2016; 194: 989–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material for Decreased peak expiratory flow rate associated with mortality in idiopathic pulmonary fibrosis: A preliminary report by Kohei Fujita, Hirotsugu Ohkubo, Akiko Nakano, Norihisa Takeda, Kensuke Fukumitsu, Satoshi Fukuda, Yoshihiro Kanemitsu, Takehiro Uemura, Tomoko Tajiri, Ken Maeno, Yutaka Ito, Tetsuya Oguri, Yoshiyuki Ozawa, Takayuki Murase and Akio Niimi in Chronic Respiratory Disease