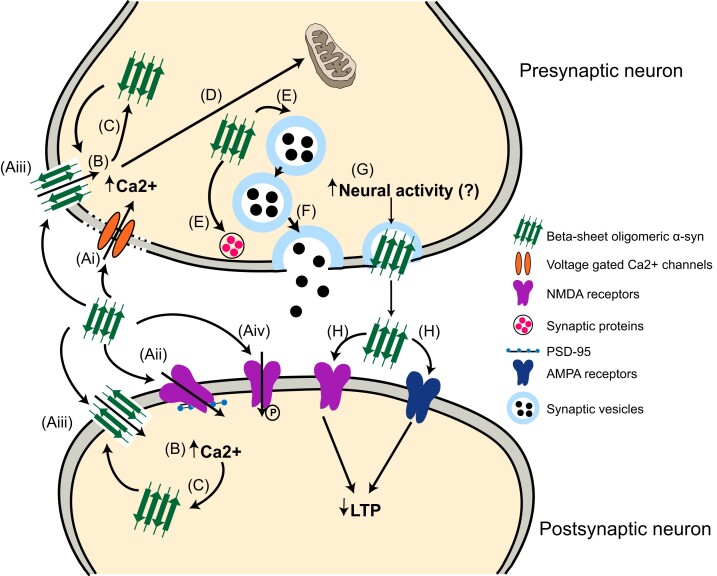
Figure 1.
Mechanisms of α-syn mediated synaptic dysfunction. (A) Oligomeric α-syn interferes with the pre- and/or postsynaptic plasma membrane integrity by dislocating the (Ai) voltage-gated Ca2+ channels, (Aii) NMDA receptors, or (Aiii) by forming pore-like structures. Additionally, (Aiv) pathological α-syn phosphorylates and activates NMDA receptors. Together, (B) these pathological α-syn interactions lead to increased intracellular Ca2+ influx. (C) Increased intracellular Ca2+ levels can further stimulate α-syn aggregation and oligomer formation. Nevertheless, (D) due to the synergistic action of elevated Ca2+ and pathological α-syn, the mitochondria undergoes oxidative stress, potentially leading to cell death. (E) Presynaptic accumulation of pathological α-syn also interferes with the synaptic pool maintenance, and synaptic proteins, entailing (F) reductions in neurotransmitter release. Neuronal activity mediates the presynaptic accumulation and extracellular release of physiological α-syn, however, it is unknown if it applies to pathological α-syn. (G) Increased neuronal activity could result in an increase in the release of extracellular oligomeric α-syn. (H) Extracellular α-syn is further proposed to impair LTP via NMDA and AMPA receptors.