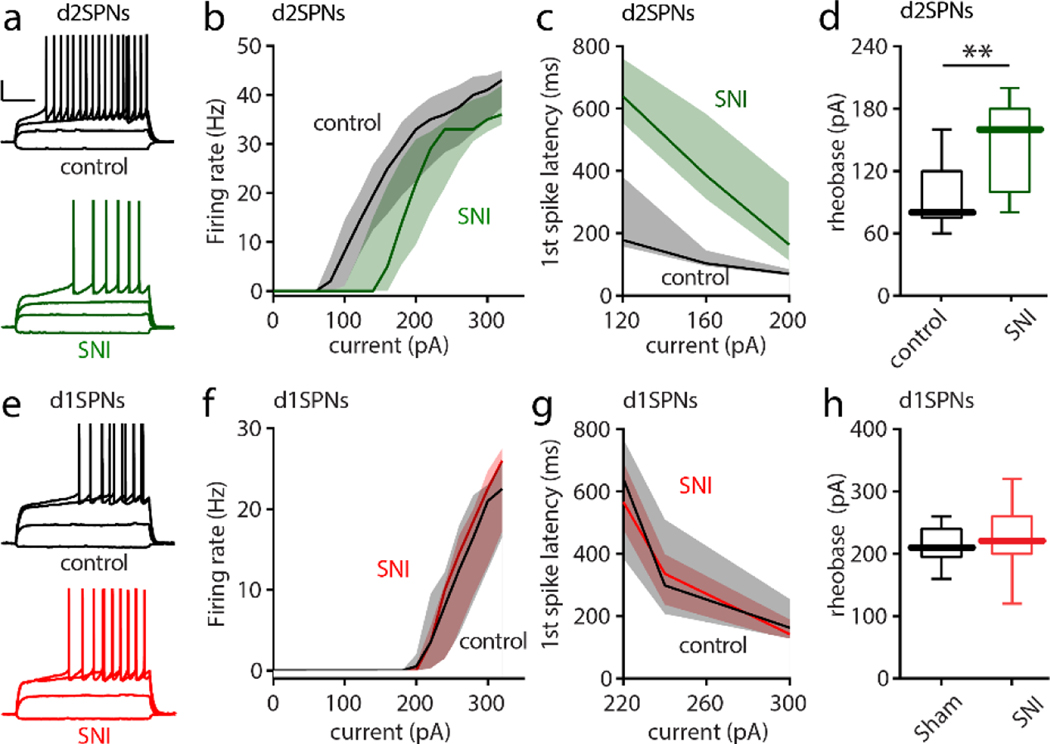
Figure 5. SNI selectively decreased the intrinsic excitability of cNAc d2SPNs.
a, Sample recordings from Sham and SNI slices (calibration bars: 20 mV, 200 ms). 5 days after Sham or SNI surgery, acute slices of cNAc were collected and input/output responses of intrinsic d2SPN excitability were obtained with depolarizing current injections (current injections: −40, 60, 120 and 160 pA). b-d, In SNI slices, intrinsic d2SPN excitability was reduced (n = 10–11 neurons from 5 mice per group; b, U = 12717, p = 0.0008) and accompanied by a longer first spike latency (c, U = 139, p < 0.0001) and elevated rheobase current (d, U = 18, p = 0.0065) in cNAc d2SPNs from SNI. e-h, the excitability of cNAc d1SPNs (input/output curve, first-spike latency and rheobase current) was unchanged by SNI (each group n=10–11 neurons from 5 mice; current injection for e : −40, 100, 220 and 260 pA; U = 15534, p = 0.6657 for f, U = 484, p = 0.8831 for g, U = 44.5, p = 0.4714 for h). Data for b,c,f,g are shown as median with shaded interquartile (quartile 1 to quartile 3); data for d,h are presented as whisker box plots displaying median, lower and upper quartiles, and whiskers representing minimum and maximum of the data. Data are analyzed by Mann–Whitney U test.