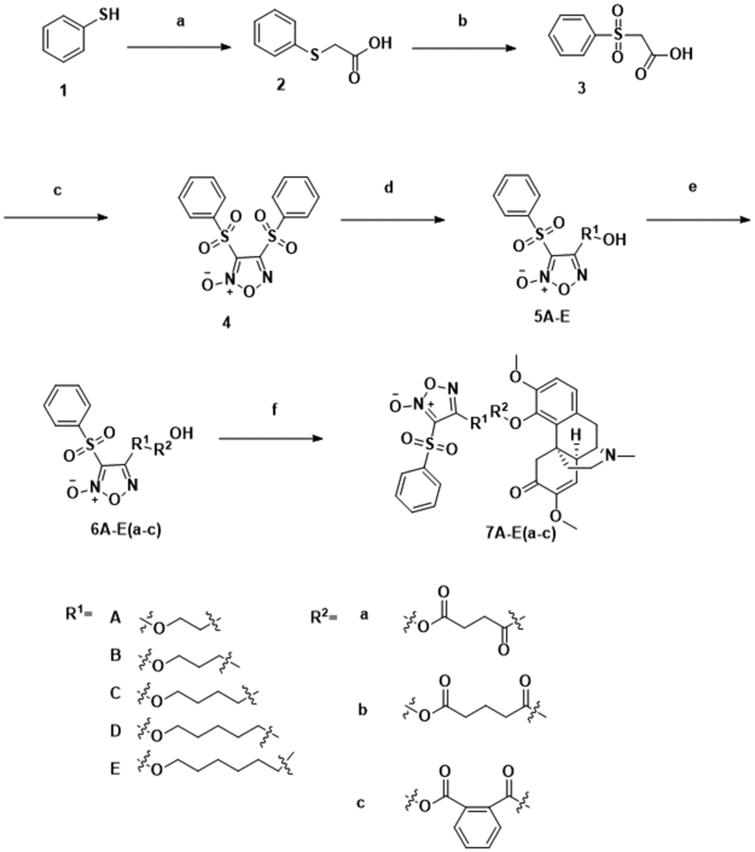
Scheme 1.
Synthesis of 1–4, 5A–E, 6A–E(a–c) and 7A–E(a–c). Reagents and conditions: (a) ClCH2COOH, NaOH (aq), reflux, 2 h; (b) 30% H2O2, AcOH, rt, 3 h; (c) fuming HNO3, 100 °C, 8 h; (d) corresponding diol, THF, 30% NaOH, 0 °C, 1 h; (e) corresponding anhydrides, TEA, DMAP, DCM, rt, 2 h; (f) DMAP, EDCI, rt, 4 h.