Abstract
Purpose
To investigate whether (1 → 3)-β-d-Glucan (BDG)-guidance shortens time to antifungal therapy and thereby reduces mortality of sepsis patients with high risk of invasive Candida infection (ICI).
Methods
Multicenter, randomized, controlled trial carried out between September 2016 and September 2019 in 18 intensive care units enrolling adult sepsis patients at high risk for ICI. Patients in the control group received targeted antifungal therapy driven by culture results. In addition to targeted therapy, patients in the BDG group received antifungals if at least one of two consecutive BDG samples taken during the first two study days was ≥ 80 pg/mL. Empirical antifungal therapy was discouraged in both groups. The primary endpoint was 28-day-mortality.
Results
339 patients were enrolled. ICI was diagnosed in 48 patients (14.2%) within the first 96 h after enrollment. In the BDG-group, 48.8% (84/172) patients received antifungals during the first 96 h after enrollment and 6% (10/167) patients in the control group. Death until day 28 occurred in 58 of 172 patients (33.7%) in the BDG group and 51 of 167 patients (30.5%) in the control group (relative risk 1.10; 95% confidence interval, 0.80–1.51; p = 0.53). Median time to antifungal therapy was 1.1 [interquartile range (IQR) 1.0–2.2] days in the BDG group and 4.4 (IQR 2.0–9.1, p < 0.01) days in the control group.
Conclusions
Serum BDG guided antifungal treatment did not improve 28-day mortality among sepsis patients with risk factors for but unexpected low rate of IC. This study cannot comment on the potential benefit of BDG-guidance in a more selected at-risk population.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00134-022-06733-x.
Keywords: Sepsis, Invasive Candida infection, Biomarker, (1 → 3)-β-d-Glucan, Antifungal therapy
Take-home message
| In this randomized multicenter clinical trial on sepsis patients we observed an earlier but also immoderate administration of antifungals when therapy was guided by (1 → 3)-β-d-glucan-guidance in comparison to culture guidance. (1 → 3)-β-d-glucan-guidance did not affect mortality but definite conclusions are hampered by an unexpectedly low rate of invasive Candida infections in this study population. |
Introduction
Sepsis is a leading cause of death in critically ill patients and is defined as a life-threatening organ dysfunction caused by a dysregulated host-response due to infection [1]. Incidence of invasive Candida infection (ICI) is increasing [2, 3]. Mortality rates reached 80% in patients with candidemia if no antifungal treatment is started within the first 24 h of septic shock [4].
Current guidelines suggest early antifungal treatment in critically ill patients with a high risk of ICI but give only little advice on the selection of appropriate patients [5–7]. (1 → 3)-β-d-Glucan (BDG) may be suitable to guide antifungal therapy as BDG serum concentrations are significantly elevated in patients with ICI [8]. BDG is a cell wall constituent of Candida spp. Previous trials suggested that BDG guided antifungal therapy is safe [9], that BDG serum concentrations may aid to stop antifungals [10, 11], and that BDG might select patients benefiting from empirical antifungal treatment [12]. Current guidelines are cautious to recommend BDG guidance to trigger antifungal treatment because of limited data [6, 7, 13]. We designed the CandiSep trial to test whether BDG-guidance shortens time to antifungal therapy and thereby reduces mortality of sepsis patients with a high risk of ICI.
Methods
Trial design
The CandiSep trial was an open, randomized, multicenter trial comparing a BDG-guided antifungal therapy versus standard of care in patients with sepsis and high risk for developing ICI conducted in 18 German intensive care units (eTable 1). The protocol has been published previously [14]. The ethics board of the Jena University Hospital, the corresponding institutional review boards of all participating centers and the Federal Institute for Drugs and Medical Devices approved the trial. The trial was registered at ClinicalTrials.gov (NCT02734550). Written informed consent was obtained from all patients or their legal representatives. The ethics committees approved a deferred consent for those patients unable to give informed consent but not having appointed a legal representative. As soon as the legal representative of the patient was available, written informed consent was obtained; otherwise, the patient was withdrawn from the study. Trial execution was overseen by an independent data and safety monitoring board.
Patient selection and randomization
Between September 2016 and September 2019, we included adult patients with sepsis and increased risk for ICI. The allowed time window between onset of sepsis and enrollment was 12 h but was extended to 24 h as of January 26, 2018 because of insufficient recruitment. Sepsis definitions were applied as reported previously [15]. However, in the light of the Sepsis-3 definitions under development when this study was designed [1], Systemic Inflammatory Response Syndrome was not required for sepsis diagnosis. Risk for ICI was defined as total parenteral nutrition, abdominal surgery within the last 7 days, previous antimicrobial therapy for more than 48 h, and previous renal replacement therapy [16]. We excluded patients with proven ICI, present or planned systemic antifungal therapy prior to inclusion, recent surgery with cardiopulmonary bypass, recent treatment with immunoglobulins, and immunosuppression. A complete list of inclusion and exclusion criteria is provided in the Supplement. After enrollment, the patients were randomized to either a BDG- or control group in a 1:1 ratio via a web-based central randomization service. The randomization list was prepared by an independent statistician via a computer-based algorithm and was stratified by study center.
Trial treatments
BDG-samples together with concomitant blood cultures were taken within 1 h after randomization and 24 h after enrollment. In the BDG-group, samples were immediately forwarded to the central laboratory. Site specific courier schedules assured that the first BDG result was available not later than 24 h after sampling. The treating physicians were informed about the BDG results via telephone and fax. Any patient with a BDG concentration of ≥ 80 pg/mL had to be treated with antifungals suitable for ICI. Antifungal treatment of patients with only one BDG concentrations ≥ 80 pg/mL was discontinued in case of blood cultures negative for Candida spp. irrespective which BDG sample was positive. Patients with BDG concentrations ≥ 80 pg/mL in both samples remained on antifungals irrespective of the blood culture results. In the control group, BDG was measured in batches. The treating physicians were blinded to the BDG results and patients were treated according to standard of care. In both groups, targeted treatment of ICI was performed according to European guidelines [17], and patients with microbiological growth of Candida spp. in any blood culture, biopsy or sample from normally sterile body fluids were treated with antifungals independent of the BDG result. Empirical antifungal therapy was discouraged in both groups.
Measurements
Blood samples were handled according to standardized procedures [14]. Briefly, serum samples for BDG-measurement were obtained within the first hour after randomization and after 24 h using BDG-free collection tubes. BDG serum concentrations were measured in a central laboratory at the Microbiology Institute of the University Hospital Erlangen, Germany using the Fungitell® assay (Associates of Cape Cod Inc., East Falmouth, MA, USA) according to the manufacturer’s instructions [14]. The Fungitell® assay is a Limulus amebocyte lysate-based test with a detection-limit at 30 pg/mL and coefficient of variation of 9.6%. Aerobic and anaerobic blood cultures were obtained by sterile venipuncture no more than 3 h after randomization if no blood cultures had already been taken up to 6 h before randomization. Additional blood samples with tubes containing ethylenediaminetetraacetic acid (EDTA) were obtained for Candida detection via polymerase chain reaction (Candida-PCR) via the same venipuncture. Microbiological samples were taken from nose/throat, skin (axillar region), rectum/feces, urine, and tracheal or bronchial secretion to determine the Candida Colonization Index (CCI) [18]. Blood cultures were repeated on the day after randomization and microbiological samples for the CCI were repeatedly obtained on day 7 and day 14 if the patient was still on the ICU. Candidemia was defined as growth of Candida spp. in at least one blood culture and ICI was defined as any microbiological proof of Candida spp. in primary sterile specimens [19]. The PCR was performed in batches in the National Reference Center for Invasive Mycoses (Jena, Germany). Candida spp. were detected by a semi-nested PCR assay amplifying the internal transcribed spacer region 2 [14]. PCR results were not reported to the treating physicians.
Outcomes
The primary outcome of the study was all-cause mortality by 28 days after inclusion. Secondary outcomes were antifungal-free survival within 28 days after inclusion, Candida colonization according to the Candida Colonization Index (CCI), time to antifungal therapy, costs of antifungal therapy, duration of organ support (including ventilation, vasopressor, and renal replacement therapy) until day 28, mean total score for Sequential Organ Failure Assessment (SOFA) calculated as the sum of daily SOFA scores divided by the number of study days on intensive care unit (ICU), ICU and hospital length of stay, ICU and hospital mortality, and frequency of adverse events.
Statistical analysis
Complete details regarding the sample size justification and statistical analysis have been reported previously [14]. Sample size was based on an estimated rate of the primary end point of 49.8% in the control group and 34.2% in the BDG group. A sample size of 156 patients per group would provide more than 80% power to show the superiority of BDG-guidance over standard of care using a two-by-two chi-square test at a two-sided alpha level of 0.05. Studies with a similar patient population have demonstrated a 10% drop-out rate [20, 21]. We therefore expected to randomize 348 patients to achieve 312 evaluable patients. An interim analysis was performed after one year of recruitment, with data available only to safety monitoring board. Based on this analysis, the data and safety monitoring board recommended to continue the trial as planned.
Study objectives were analyzed in the intention-to-treat population (ITT), which consisted of all randomized patients with informed consent. The primary objective was analyzed by using the chi-square test. Kaplan–Meier estimates and log rank tests were also applied. Twenty-eight day mortality was also analyzed in a per-protocol analysis and for the following pre-defined subgroups: septic shock at randomization, CCI ≥ 0.5, Candida-PCR positive, blood culture positive for Candida spp., BDG serum concentration ≥ 80 pg/mL in both samples, ≥ 2 risk factors for ICI. Patients were excluded for the per-protocol population if antifungal therapy deviated from the recommendation based on the BDG results (BDG-group), and if the patients received systemic antifungal therapy in absence of proven ICI (control group). Secondary objectives were analyzed according to their scales with the Chi-square or Fisher’s ex!ct test and Mann–Whitney-U-test, respectively.
Results
Patients
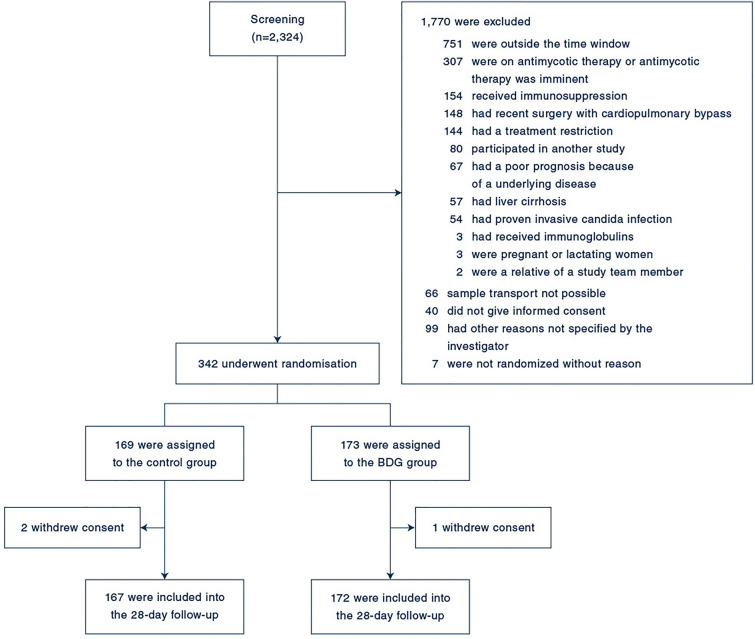
Patients were recruited from September 2016 to September 2019. We identified 2324 patients eligible of whom 342 underwent randomization. Seventy-eight patients were enrolled before the amendment. Three patients withdrew informed consent resulting in 339 patients, of whom 172 patients in the BDG group and 167 patients in the control group were included in the analysis of the primary endpoint (Fig. 1). Baseline characteristics were well balanced between the two groups (Table 1) with recent abdominal surgery being the most frequent ICI risk factor. Initial blood cultures were positive for Candida spp. in 15 patients (4.4%). ICI was diagnosed in 48 patients (14.2%) within the first 96 h after enrollment (see eTable 2 for characteristics of patients with ICI). The most frequent pathogen was Candida albicans (see eTable 3 for characteristics of ICI). Six patients (1.8%) were positive for Candida spp. in the PCR but only three of these patients had microbiological proof of Candida spp. and were considered having ICI. Sensitivity and specificity of two positive BDG results to predict candidemia were 64.3% and 63.7% and to predict ICI diagnosis within the first 96 h were 54.4% and 65.2%, respectively (see eTable 4 for association of BDG results and ICI). The per-protocol population consisted of 101 patients in the BDG- and 127 patients in the control-group (see eTable 5 for characteristics of patients).
Fig. 1.
Participant flow in the CandiSep-trial. Categories of screening failures were not mutually exclusive; participants could have more than one reason. BDG group: (1 → 3)-β-d-glucan-guided group
Table 1.
Characteristics of the patients at baseline
| BDG group n = 173 |
Control group n = 169 |
|
|---|---|---|
| Demographics | ||
| Age—years | 70 (58–78) | 71 (60–78) |
| Male sex—no (%) | 117 (67.6) | 105 (62.1) |
| Body mass index—kg/m2 | 26.1 (23.2–29.4) | 26 (23.1–29.7) |
| Charlson Comorbidity Index | 2 (0–3.5) | 2 (1–5) |
| APACHE II Score | 20.5 (16–25) | 20 (17–27) |
| SAPS II | 47 (39–58) | 50 (40–62) |
| SOFA-Score | 12 (10–15) | 13 (11–15) |
| Lactate levels—mmol/l | 2.8 (1.9–4.7) | 2.7 (1.7–4.5) |
| Procalcitonin levels—ng/mL | 6.5 (1.9–23.2) | 6.8 (1.8–22) |
| Patients on antimicrobial agents—no (%) | 170 (98.3) | 165 (97.6) |
| Septic shock—no (%) | 152 (87.9) | 157 (92.9) |
| Patients on mechanical ventilation—no (%) | 139 (80.3) | 138 (82.1) |
| Hospital stay before randomization—days | 5.3 (1.1–11.3) | 5.3 (1.4–10.4) |
| ICU stay before randomization—days | 0.8 (0.5–2.8) | 0.7 (0.5–1.8) |
| Time to randomization (hours) | 13.9 (8.1–20.4) | 12.6 (8.5–17.4) |
| Risk factors for invasive candida infection—no (%) | ||
| Total parenteral nutrition | 11 (6.4) | 13 (7.7) |
| Recent abdominal surgery | 117 (67.6) | 119 (70.4) |
| Recent antimicrobial therapy > 48 h | 78 (45.1) | 73 (43.2) |
| Preexisting renal replacement therapy | 15 (8.7) | 20 (11.8) |
| Sepsis etiology—no (%) | ||
| Pulmonary | 38 (22) | 40 (24) |
| Abdominal | 104 (60.1) | 109 (65.3) |
| Blood | 7 (4) | 4 (2.4) |
| Wound, skin or soft tissue | 20 (11.6) | 17 (10.2) |
| Urinary | 7 (4) | 7 (4.2) |
| Other | 13 (7.5) | 13 (7.8) |
| no infection identified | 25 (14.5) | 17 (10.2) |
| Initial blood culture positive for Candida spp.—no (%) | 8 (4.6) | 7 (4.1) |
| Initial PCR positive for Candida spp.—no (%) | 2 (1.2) | 4 (2.4) |
| Invasive Candida infection—no (%) | 25 (14.5) | 23 (13.8) |
| (1,3)-β-d-Glucan levels (baseline)—pg/mL | 73 (30–232) | 61 (30–226) |
| (1,3)-β-d-Glucan levels (day 1)—pg/mL | 58 (30–199) | 56 (30–194) |
Risk factors and sepsis etiology: multiple occurrences possible. Median (IQR) or number of patients (%) are displayed
ICU intensive care unit, APACHE acute physiology and chronic health evaluation, PCR polymerase chain reaction, SAPS simplified acute physiology score, SOFA sequential organ failure assessment
Trial regimens
In the BDG-group, 84 of 172 (48.8%) patients received antifungal therapy, 74 of 172 (43%) patients because of elevated BDG concentrations, 3 (1.7%) patients because of microbiological findings and 7 (4.1%) patients were treated empirically. During the same period, 10 out of 167 (6%) patients in the control group received empirical antifungal treatment, 9 (5.4%) patients because of microbiologically proven candidiasis, and one (0.6%) patient without specified reason. Protocol deviations are listed in the Supplement.
Primary outcome
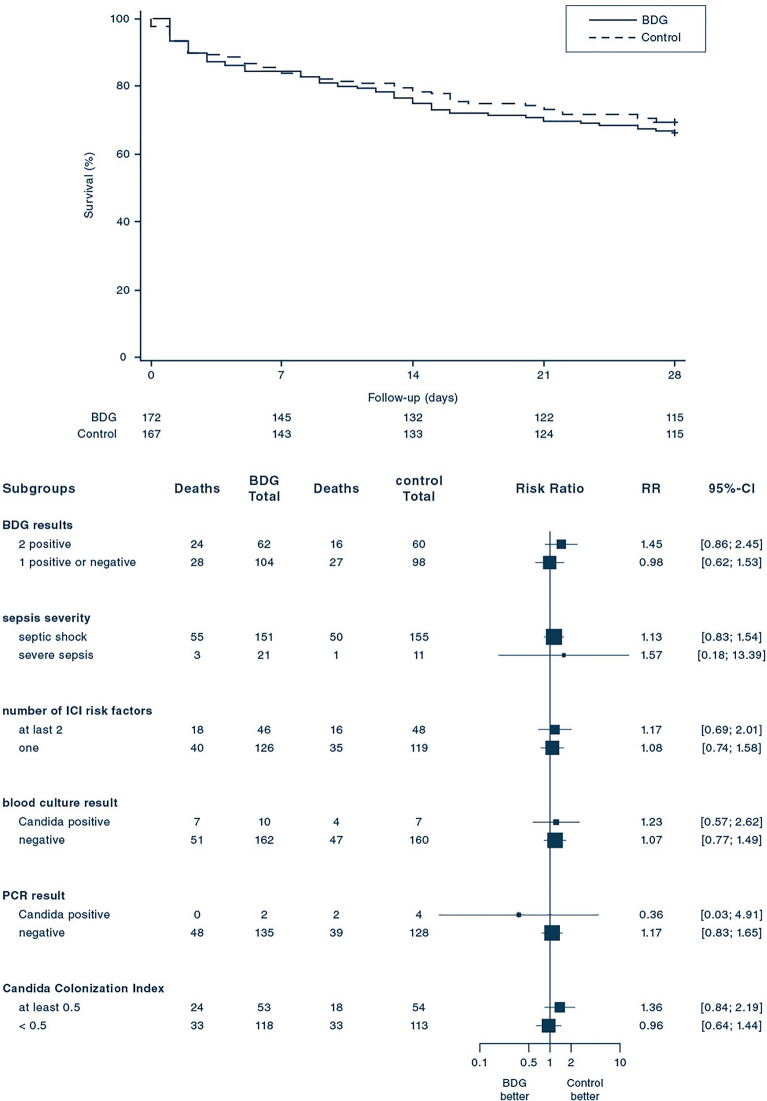
After 28 days of randomization, 58 of 172 (33.7%) patients assigned to the BDG group and 51 of 167 (30.5%) patients assigned to the control group had died [relative risk, 1.10; 95% confidence interval (CI) 0.80–1.51; p = 0.53; Table 2]. There was no significant difference between the groups concerning the rate of death in the time-to-event-analysis during the 28 days after randomization (Fig. 2). The analysis of pre-specified subgroups yielded similar results with no subgroup showing a benefit of BDG-guidance. In the per protocol analysis, 24 out of 96 (25.0%) subjects died until day 28 in the BDG group and 41 out of 126 (32.5%, p = 0.22) patients in the control group (eFigure 1). In an un-prespecified post-hoc analysis on patients having ICI, 12 of 25 (48%) patients of the BDG group and 9 of 23 patients (39.1%) of the control group died until day 28 (relative risk, 1.23; 95% CI, 0.64–2.36, p = 0.54).
Table 2.
Primary and secondary outcomes
| Outcome | BDG-group n = 172 |
Control-group n = 167 |
Relative risk (95% CI) |
p value |
|---|---|---|---|---|
| Primary outcome | ||||
| 28-Day all-cause mortality—no (%) | 58 (33.7) | 51 (30.5) | 1.1 (0.8–1.51) | 0.53 |
| Secondary outcomes | ||||
| Hospital mortality—no (%) | 59 (34.5) | 60 (35.9) | 0.96 (0.71–1.29) | 0.78 |
| Hospital length of stay—days | 25.5 (16–41) | 28 (17–48) | NA | 0.37 |
| ICU mortality- no (%) | 48 (27.7) | 47 (27.8) | 1 (0.7–1.41) | 0.99 |
| ICU length of stay—days | 11 (6–20) | 11 (4–22) | NA | 0.70 |
| Antifungal free survival at day 28—no (%) | 52 (30.2) | 87 (52.1) | 2.97 (2.1–4.2) | < 0.01 |
| Time to antifungal therapy—days | 1.1 (1–2.2) | 4.4 (2–9.1) | NA | < 0.01 |
| Costs of antifungal therapy—Euro | 4451 (1385–6923) | 2800 (989–7097) | NA | 0.52 |
| Candida Colonization Index | ||||
| At randomization | 0.20 (0–0.33) | 0.2 (0–0.4) | NA | 0.69 |
| At day 1 | 0 (0–0.67) | 0 (0–1) | NA | 0.66 |
| At day 7 | 0.25 (0–0.5) | 0.25 (0; 0.5) | NA | 0.22 |
| At day 14 | 0.2 (0–0.33) | 0.25 (0.08–0.4) | NA | 0.14 |
| Total SOFA | 10.5 (8.2–14.3) | 10.4 (8.2–13.4) | NA | 0.42 |
| Vasopressor free days—days | 20 (3–25) | 20 (3–26) | NA | 0.40 |
| Ventilator free days—days | 16 (2–25) | 15 (2–27) | NA | 0.51 |
| Renal replacement free days—days | 27 (9–29) | 27.5 (9–29) | NA | 0.92 |
NA not applicable, IQR interquartile range, ICU intensive care unit, SOFA sequential organ failure assessment
Fig. 2.
Rate of survival and Risk of Death at day 28, according to subgroups. Upper panel shows Kaplan–Meier estimates of the survival rate according to the (1 → 3)-β-d-glucan-guided (BDG) and the control-group. The p value of 0.55 was calculated with the log-rank-test. Lower panel shows the relative risk (RR) of death at day 28 in the prespecified subgroups. The size of the square representing the relative risk reflects the relative numbers in each subgroup, and horizontal bars represent 95% confidence intervals
Secondary outcomes
Secondary outcomes are shown in Table 2. Patients of the BDG group received antifungals within a median time of 1.1 [interquartile range (IQR) 1.0–2.2] days while time to antifungal therapy was 4.4 (IQR: 2.0–9.1, p < 0.01) days in the control group. In total, there was a higher frequency of antifungal therapy in the BDG group (99 of 172 patients, 57.6%) compared to the control group (46 of 167 patients, 27.5%; Fig. 3). Median duration of antifungal therapy was 10.5 (IQR: 5.6–14.7) days in the BDG group versus 10.0 (IQR 4.2–15.4, p = 0.69) days in the control group. Antifungal free survival at day 28 was significantly lower in the BDG group than in the control group (30.2% vs. 52.1%, p < 0.01) with an odds ratio of 2.97 (95% CI 2.1–4.2). This was associated with higher per patient costs of antifungal therapy in the BDG (4451 €, IQR 1385–6923) compared to the control group (2800 €, IQR 989–7097) but this difference did not reach statistical significance (p = 0.52). Candida colonization was generally low in the study population (median CCI: 0.2 in both groups) and did not change throughout the trial. There was no overt safety issue with BDG-guidance (see Supplement).
Fig. 3.
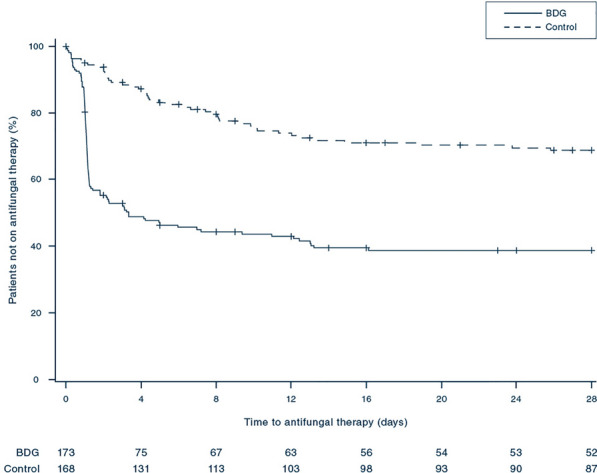
Rate of patients not on antifungal therapy. The figure shows Kaplan–Meier estimates of the rate of patients not on antifungal therapy according to the (1 → 3)-β-d-glucan-guided (BDG) and the control-group. The p value of < 0.01 was calculated with the Log-rank-test
Discussion
In this multicenter randomized trial, BDG guidance in critically ill patients with sepsis or septic shock and at high risk for ICI resulted in a more frequent and earlier initiation of antifungal therapy but did not improve 28-day mortality. None of the pre-defined subgroups showed a benefit from the BDG guided early antifungal therapy. However, we observed a higher antifungal free survival at day 28 in the control group compared to the BDG-group. Thus, BDG-guided patients were more likely to receive antifungal therapy and thereby might generate higher costs for antifungal therapy.
International guidelines [5–7] recommend antifungal therapy in critically ill patients without proven Candida infection but with inherent risk factors. This recommendation is based on the high risk of developing ICI [18, 22] and the high mortality rate in case of untreated ICI [4] in this patient population. Some studies suggested a benefit for risk-based antifungal therapy [23, 24] while other randomized controlled trials did not show a benefit for such an approach [12, 25–27]. Our study now demonstrates that a BDG-guided initiation of early antifungal therapy was not superior to a wait-and-see culture-based approach in a sepsis population with a high risk of ICI.
Specificity of BDG was low in our study. Various factors have been discussed for triggering false positive BDG results including administration of antimicrobials [28], blood products including albumin [29], and immunoglobulins [30], all of which may be applied in the care of sepsis patients. However, significance of these factors is not undisputed [31]. Previous abdominal surgery was the most prevalent risk factor predisposing for ICI in our trial. However, open gut surgery results in elevated serum BDG concentrations for up to 5 days which might have impacted our BDG-measurements [32]. Although BDG was eliminated within 4 days in pediatric patients [33], animal experiments described a BDG-clearance of 9 days [34]. The most recent Cochrane analysis concluded that it remains unclear whether BDG can identify ICI early [35]. This lack of specificity likely caused an overuse of antifungals in the BDG-group as many patients without microbiological proof of ICI were treated without having a benefit. We aimed to improve BDG-specificity by excluding patients treated with immunoglobulins [30] or cardiopulmonary bypass surgery [36] and by requiring two positive BDG measurements from two consecutive days [37, 38]. Although a BDG cut-off of 80 pg/mL has been successfully used to diagnose candidemia and intraabdominal candidiasis [8, 39, 40], a higher cut-off such as 200 pg/mL has been suggested to increase specificity [41]. Sensitivity was only 54.3% in our study. A sensitivity as low as 20% has been observed in several studies [35] and may be associated with a low pathogen load [39]. Current data rather suggest that BDG can be used as a surrogate for withdrawal of empirically started antifungal treatment rather than early initiation [10, 11, 42].
Our sample size calculation was based on a mortality rate of 49.8% considering the high-risk profile of this patient population [14]. However, the mortality rate in the control group was 30.5% and, thereby, lower than expected. This might be partly explained by the high frequency of C. albicans-infections being associated with a lower mortality than infections with C. glabrata or C. krusei [43]. In addition, our inclusion criteria aimed for a patient population with a high risk for ICI, but surprisingly, we only observed fifteen cases of proven candidemia, 48 patients with ICI and a low Candida colonization rate. A low rate of Candida colonization is associated with lower risk for developing ICI [44]. This was accompanied by a low positivity rate of the Candida-PCR. Therefore, the inclusion criteria did not result in a pre-selection of a population with high risk of ICI, and BDG may thus not unfold its full diagnostic benefit. Interestingly, this observation does not align with studies with a similar patient profile but a higher proportion of patients with candidemia [11, 40, 45]. Prediction of ICI solely based on risk factors may be too inaccurate to select patients for antifungal therapy.
Strengths and Limitations
The strengths of our trial include the randomized, controlled design, and its multicenter approach. The patients were efficiently enrolled shortly after onset of sepsis. Randomization resulted in an equal distribution of possible confounders between the two groups. Our trial achieved a sufficient separation between the groups regarding timing and frequency of antifungal therapy. Nevertheless, the trial also had limitations. Quality of BDG measurement required a central lab. Compared to in-house measurement, this approach resulted in a delay of measurement and reporting despite an established express delivery service. We increased the time window for recruitment from 12 to 24 h to facilitate sufficient enrollment. Both factors possibly inflicted a delay in antifungal therapy to the disadvantage of the BDG group. The strict time window and the exclusion criteria caused an enrolment rate of 14.7% of the screened patients. Such a low rate might affect the generalizability of the trial. We had a significant number of protocol deviations mainly addressing the duration of the antifungal therapy in the BDG group. The lower mortality in the BDG-group compared to the control group in the per-protocol analysis suggests that an improvement in algorithm adherence might have impacted the outcome. However, a per-protocol-analysis does not maintain internal validity as obtained by randomization and adequate sample size. Frequency of ICI was unexpectedly low. BDG-guidance might perform better in a study population with a higher rate of ICI. The investigated intervention did not allow for blinding. Therefore, this trial may be affected by performance and detection bias.
Conclusion
Early administration of antifungal therapy guided by serum BDG did not provide an advantage over standard of care as measured by 28-day mortality among critically ill patients with sepsis. These data do not support BDG guidance for initiation of antifungal therapy in this patient population if the observed rate of ICI is reasonably low. A possible benefit of serial BDG measurement in a more selected population at risk needs to be addressed in future studies.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The SepNet study group: Ulrich Jaschinski (University Hospital Augsburg, Dept. of Anesthesiology and Surgical Intensive Care Medicine, Augsburg), Christian Putensen (University Hospital Bonn Division of Intensive Care Medicine, Dept. of Anesthesiology and Intensive Care Medicine, Bonn), Klaus Kogelmann, Matthias Drüner (Hospital Emden, Dept. of Anesthesiology and Intensive Care Medicine, Emden), Ixchel Castellanos, Stefanie Schmidt, Andreas Wehrfritz, Diana Kränzlein (University Hospital Erlangen, Dept. of Anesthesiology, Erlangen), Jürgen Held (Universitätsklinikum Erlangen und Friedrich-Alexander-Universität (FAU) Erlangen-Nürnberg, Mikrobiologisches Institut - Klinische Mikrobiologie, Immunologie und Hygiene, Erlangen), Kai Zacharowski, Haitham Mutlak, Simone Lindau, Carolin Wiedenbeck (University Hospital Frankfurt, Dept. of Anesthesiology, Intensive Care Medicine, and Pain Therapy, Frankfurt), Onnen Mörer (Dept. of Anesthesiology, University Medical Center, Georg-August-University Göttingen, Göttingen), Sven-Olaf Kuhn, Matthias Gründling (University Hospital Greifswald, Dept. of Anesthesiology and Intensive Care Medicine, Greifswald), Stephan Kluge, Geraldine de Heer, Dominik Jarczak (University Hospital Hamburg-Eppendorf, Dept. of Intensive Care Medicine, Hamburg), Johann Motsch, Daniel Richter, Markus A. Weigand (University Hospital Heidelberg, Dept. of Anesthesiology, Heidelberg), Frank Bloos, Michael Bauer, Daniel Thomas-Rüddel (Jena University Hospital, Dept. of Anesthesiology and Intensive Care Medicine, Center for Sepsis Control & Care (CSCC), Jena), Peter Schlattmann, Thomas Lehmann (Jena University Hospital, Institute of Medical Statistics, Computer Sciences and Data Science, Jena), Norbert Weiler, Dirk Schädler (University Medical Center Kiel, Dept. of Anesthesiology and Intensive Care Medicine, Kiel), Oliver A. Cornely (University of Cologne, Faculty of Medicine and University Hospital Cologne, Department I of Internal Medicine, Excellence Center for Medical Mycology (ECMM), Cologne, Germany; University of Cologne, Faculty of Medicine and University Hospital Cologne, Chair Translational Research, Cologne Excellence Cluster on Cellular Stress Responses in Aging-Associated Diseases (CECAD), Cologne, Germany; University of Cologne, Faculty of Medicine and University Hospital Cologne, Clinical Trials Centre Cologne (ZKS Köln), Cologne, Germany; German Centre for Infection Research (DZIF), Partner Site Bonn-Cologne, Cologne, Germany), Philipp Simon, Gunther Hempel (University of Leipzig Medical Centre, Dept. of Anesthesiology and Intensive Care Medicine, Leipzig), Raphael Weiss, Alexander Zarbock (University Hospital Münster, Dept. of Anesthesiology, Surgical Intensive Care Medicine and Pain Therapy, Münster), Ulf Günther, Georg Rohe (Klinikum Oldenburg, University Clinic of Anesthesiology, Intensive Care, Emergency Medicine and Pain Therapy, Oldenburg), Andreas Weyland (Carl von Ossietzky Universität Oldenburg, Research Center Neurosensory Science, Oldenburg), Oliver Kurzai, Grit Walter (Julius Maximilians University Würzburg, Institute for Hygiene and Microbiology, Würzburg), Patrick Meybohm, Philipp Helmer (University Hospital Wuerzburg , Dept. of Anesthesiology, Emergency and Pain Medicine, Würzburg).
Author contributions
FB and DT-R worked out the underlying scientific concept of the study. FB, JH, PS, and DT-R designed the study. FOC was the scientific advisor of the study accompanying all phases of the trial. JH was responsible for implementing and running the (1,3)-β-d-glucan measurement for the trial. OK was responsible for implementing and running the Candida-PCR for the trial. SK, PS, KK, GH, SOK, DJ, JM, GH, NW, AW, MD, MG, PM, DR, UJ, OM, UG, DS, RW, CP, IC, MB were responsible for the local study infrastructure and recruitment at the study sites. PS was responsible for data management and statistical analysis of the trial. The first draft was written by FB, JH, and DT-R. All authors read and commented on the drafts and approved the final manuscript.
Funding
Open Access funding enabled and organized by Projekt DEAL. This trial was supported by a Grant (01EO1502) to the Jena University Hospital provided by the German Federal Ministry of Education and Research. The Fungitell®-assay was provided free of charge for this study by Associates of CapeCod, Inc., USA. Prof. Bauer is funded by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) under Germany’s Excellence Strategy–EXC 2051–Project-ID 390713860. Prof. Putensen is funded by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation)–Project-ID PU219/2-3.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Conflicts of interest
FB received honoraria for an expert board meeting by Baxter. Prof. Cornely reports grants or contracts from Amplyx, Basilea, BMBF, Cidara, DZIF, EU-DG RTD (101037867), F2G, Gilead, Matinas, MedPace, MSD, Mundipharma, Octapharma, Pfizer, Scynexis; Consulting fees from Amplyx, Biocon, Biosys, Cidara, Da Volterra, Gilead, Matinas, MedPace, Menarini, Molecular Partners, MSG-ERC, Noxxon, Octapharma, PSI, Scynexis, Seres; Honoraria for lectures from Abbott, Al-Jazeera Pharmaceuticals, Astellas, Grupo Biotoscana/United Medical/Knight, Hikma, MedScape, MedUpdate, Merck/MSD, Mylan, Pfizer; Payment for expert testimony from Cidara; Participation on a Data Safety Monitoring Board or Advisory Board from Actelion, Allecra, Cidara, Entasis, IQVIA, Jannsen, MedPace, Paratek, PSI, Shionogi; A pending patent currently reviewed at the German Patent and Trade Mark Office; Other interests from DGHO, DGI, ECMM, ISHAM, MSG-ERC, Wiley. JH received grants and speaker honoraria from Pfizer, speaker honoraria from Gilead and consumables/test kits from Associates of Cape Cod. SK received research support from Cytosorbents and Daiichi Sankyo. He also received lecture fees from Astra, Bard, Baxter, Biotest, Cytosorbents, Daiichi Sankyo, Fresenius Medical Care, Gilead, Mitsubishi Tanabe Pharma, MSD, Pfizer, Philips and Zoll. He received consultant fees from Fresenius, Gilead, MSD and Pfizer. KK received honoraria for lecturing from Cytosorbents, Fresenius and Sedana. CP received lecture fees from Astra, C.R.Bard, Baxter, Biotest, Cytosorbents, Daiichi Sankyo, Fresenius, Gilead, Mitsubishi Tanabe Pharma, MSD, Pfizer, Philips and Zoll. He received consultant fees from Bayer, Fresenius, Gilead, MSD and Pfizer. He also received consultant fees from Messer, Pluristem, and Sedana and received lecture fees from Dräger Med. Inc. and Medronic. OM received honoraria for lectures during workshops on hemodynamic monitoring, supported by Pulsion (Maquet Critical Care) and for two lectures during industrial sessions at national congresses (HillRom, HepaWash); Unrestricted Research Grant from CSL Behring. DR has received support for attending meetings and/or travels from Gilead Sciences Inc., MSD, Pfizer. AW reported receiving honoraria for lecturing from Getinge and receiving personal fees from TEVA for consulting. No other disclosures were reported.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. The study was approved by the Ethics Committee of the Jena University Hospital and by the responsible Ethics Committees of the participating study centers.
Consent to participate
Written informed consent was obtained from all patients or their legal representatives, if available. In cases where consent could neither be obtained from the patient nor a legal representative in time before enrollment, the local ethics committee approved a delayed consent process. During this process the patient’s inability to provide consent was confirmed by an independent physician. As soon as the legal representative of the patient was available, written informed consent was immediately obtained, otherwise the patient was withdrawn from the study and all study procedures were ended.
Consent for publication
Not applicable.
Footnotes
A complete list of members of the SepNet study group is provided in the Acknowledgements.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Frank Bloos, Email: frank.bloos@med.uni-jena.de.
the SepNet Study Group:
Ulrich Jaschinski, Christian Putensen, Matthias Drüner, Ixchel Castellanos, Stefanie Schmidt, Andreas Wehrfritz, Diana Kränzlein, Jürgen Held, Kai Zacharowski, Haitham Mutlak, Simone Lindau, Carolin Wiedenbeck, Onnen Mörer, Sven-Olaf Kuhn, Matthias Gründling, Stephan Kluge, Geraldine de Heer, Dominik Jarczak, Johann Motsch, Daniel Richter, Markus A. Weigand, Frank Bloos, Michael Bauer, Daniel Thomas-Rüddel, Peter Schlattmann, Thomas Lehmann, Norbert Weiler, Dirk Schädler, Oliver A. Cornely, Philipp Simon, Gunther Hempel, Raphael Weiss, Alexander Zarbock, Ulf Günther, Georg Rohe, Andreas Weyland, Oliver Kurzai, Grit Walter, Patrick Meybohm, and Philipp Helmer
References
- 1.Singer M, Deutschman CS, Seymour CW, et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3) JAMA. 2016;315:801–810. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bassetti M, Giacobbe DR, Vena A, et al. Incidence and outcome of invasive candidiasis in intensive care units (ICUs) in Europe: results of the EUCANDICU project. Crit Care. 2019;23:219. doi: 10.1186/s13054-019-2497-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Koehler P, Stecher M, Cornely OA, et al. Morbidity and mortality of candidaemia in Europe: an epidemiologic meta-analysis. Clin Microbiol Infect. 2019;25:1200–1212. doi: 10.1016/j.cmi.2019.04.024. [DOI] [PubMed] [Google Scholar]
- 4.Kollef M, Micek S, Hampton N, Doherty JA, Kumar A. Septic shock attributed to Candida infection: importance of empiric therapy and source control. Clin Infect Dis. 2012;54:1739–1746. doi: 10.1093/cid/cis305. [DOI] [PubMed] [Google Scholar]
- 5.Evans L, Rhodes A, Alhazzani W, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock 2021. Intensive Care Med. 2021 doi: 10.1007/s00134-021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pappas PG, Kauffman CA, Andes DR, et al. Clinical practice guideline for the management of candidiasis: 2016 update by the Infectious Diseases Society of America. Clin Infect Dis. 2016;62:e1–50. doi: 10.1093/cid/civ933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Martin-Loeches I, Antonelli M, Cuenca-Estrella M, et al. ESICM/ESCMID task force on practical management of invasive candidiasis in critically ill patients. Intensive Care Med. 2019;1:1. doi: 10.1007/s00134-019-05599-w. [DOI] [PubMed] [Google Scholar]
- 8.Ostrosky-Zeichner L, Alexander BD, Kett DH, et al. Multicenter clinical evaluation of the (1 → 3) beta-d-glucan assay as an aid to diagnosis of fungal infections in humans. Clin Infect Dis. 2005;41:654–659. doi: 10.1086/432470. [DOI] [PubMed] [Google Scholar]
- 9.Hanson KE, Pfeiffer CD, Lease ED, et al. β-d-glucan surveillance with preemptive anidulafungin for invasive candidiasis in intensive care unit patients: a randomized pilot study. PLoS ONE. 2012;7:e42282. doi: 10.1371/journal.pone.0042282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rouzé A, Loridant S, Poissy J, et al. Biomarker-based strategy for early discontinuation of empirical antifungal treatment in critically ill patients: a randomized controlled trial. Intensive Care Med. 2017;43:1668–1677. doi: 10.1007/s00134-017-4932-8. [DOI] [PubMed] [Google Scholar]
- 11.De Pascale G, Posteraro B, D’Arrigo S, et al. (1,3)-β-d-Glucan-based empirical antifungal interruption in suspected invasive candidiasis: a randomized trial. Crit Care. 2020;24:550. doi: 10.1186/s13054-020-03265-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Timsit JF, Azoulay E, Schwebel C, et al. Empirical micafungin treatment and survival without invasive fungal infection in adults with ICU-acquired sepsis, candida colonization, and multiple organ failure: the EMPIRICUS randomized clinical trial. JAMA. 2016;316:1555–1564. doi: 10.1001/jama.2016.14655. [DOI] [PubMed] [Google Scholar]
- 13.O’Leary RA, Einav S, Leone M, Madách K, Martin C, Martin-Loeches I. Management of invasive candidiasis and candidaemia in critically ill adults: expert opinion of the European Society of Anaesthesia Intensive Care Scientific Subcommittee. J Hosp Infect. 2018;98:382–390. doi: 10.1016/j.jhin.2017.11.020. [DOI] [PubMed] [Google Scholar]
- 14.Bloos F, Held J, Schlattmann P, et al. (1,3)-β-d-glucan-based diagnosis of invasive Candida infection versus culture-based diagnosis in patients with sepsis and with an increased risk of invasive Candida infection (CandiSep): study protocol for a randomized controlled trial. Trials. 2018;19:472. doi: 10.1186/s13063-018-2868-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bloos F, Trips E, Nierhaus A, et al. Effect of sodium selenite administration and procalcitonin-guided therapy on mortality in patients with severe sepsis or septic shock: a randomized clinical trial. JAMA Intern Med. 2016;176:1266–1276. doi: 10.1001/jamainternmed.2016.2514. [DOI] [PubMed] [Google Scholar]
- 16.Thomas-Rüddel D, Schlattmann P, Pletz M, Kurzai O, Bloos F. Risk factors for invasive candida infection in critically ill patients—a systematic review and meta-analysis. Chest. 2021 doi: 10.1016/j.chest.2021.08.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cornely OA, Bassetti M, Calandra T, et al. ESCMID* guideline for the diagnosis and management of Candida diseases 2012: non-neutropenic adult patients. Clin Microbiol Infect. 2012;18(Suppl 7):19–37. doi: 10.1111/1469-0691.12039. [DOI] [PubMed] [Google Scholar]
- 18.Pittet D, Monod M, Suter PM, Frenk E, Auckenthaler R. Candida colonization and subsequent infections in critically ill surgical patients. Ann Surg. 1994;220:751–758. doi: 10.1097/00000658-199412000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.De Pauw B, Walsh TJ, Donnelly JP, et al. Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clin Infect Dis. 2008;46:1813–1821. doi: 10.1086/588660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brunkhorst FM, Engel C, Bloos F, et al. Intensive insulin therapy and pentastarch resuscitation in severe sepsis. N Engl J Med. 2008;358:125–139. doi: 10.1056/NEJMoa070716. [DOI] [PubMed] [Google Scholar]
- 21.Brunkhorst FM, Oppert M, Marx G, et al. Effect of empirical treatment with moxifloxacin and meropenem vs meropenem on sepsis-related organ dysfunction in patients with severe sepsis: a randomized trial. JAMA. 2012;307:2390–2399. doi: 10.1001/jama.2012.5833. [DOI] [PubMed] [Google Scholar]
- 22.Blumberg HM, Jarvis WR, Soucie JM, et al. Risk factors for candidal bloodstream infections in surgical intensive care unit patients: the NEMIS prospective multicenter study. The National Epidemiology of Mycosis Survey. Clin Infect Dis. 2001;33:177–186. doi: 10.1086/321811. [DOI] [PubMed] [Google Scholar]
- 23.Piarroux R, Grenouillet F, Balvay P, et al. Assessment of preemptive treatment to prevent severe candidiasis in critically ill surgical patients. Crit Care Med. 2004;32:2443–2449. doi: 10.1097/01.ccm.0000147726.62304.7f. [DOI] [PubMed] [Google Scholar]
- 24.Shan YS, Sy ED, Wang ST, Lee JC, Lin PW. Early presumptive therapy with fluconazole for occult Candida infection after gastrointestinal surgery. World J Surg. 2006;30:119–126. doi: 10.1007/s00268-005-7807-z. [DOI] [PubMed] [Google Scholar]
- 25.Ostrosky Zeichner L, Shoham S, Vazquez J, et al. MSG-01: a randomized, double-blind, placebo-controlled trial of caspofungin prophylaxis followed by preemptive therapy for invasive candidiasis in high-risk adults in the critical care setting. Clin Infect Dis. 2014;58:1219–1226. doi: 10.1093/cid/ciu074. [DOI] [PubMed] [Google Scholar]
- 26.Knitsch W, Vincent J-L, Utzolino S, et al. A randomized, placebo-controlled trial of preemptive antifungal therapy for the prevention of invasive candidiasis following gastrointestinal surgery for intra-abdominal infections. Clin Infect Dis. 2015;61:1671–1678. doi: 10.1093/cid/civ707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schuster MG, Edwards JE, Sobel JD, et al. Empirical fluconazole versus placebo for intensive care unit patients: a randomized trial. Ann Intern Med. 2008;149:83–90. doi: 10.7326/0003-4819-149-2-200807150-00004. [DOI] [PubMed] [Google Scholar]
- 28.Liss B, Cornely OA, Hoffmann D, Dimitriou V, Wisplinghoff H. 1,3-β-d-Glucan contamination of common antimicrobials. J Antimicrob Chemother. 2016;71:913–915. doi: 10.1093/jac/dkv419. [DOI] [PubMed] [Google Scholar]
- 29.Liss B, Cornely OA, Hoffmann D, Dimitriou V, Wisplinghoff H. 1,3-β-d-Glucan concentrations in blood products predict false positive post-transfusion results. Mycoses. 2016;59:39–42. doi: 10.1111/myc.12432. [DOI] [PubMed] [Google Scholar]
- 30.Egger M, Prüller F, Raggam R, et al. False positive serum levels of (1–3)-β-d-glucan after infusion of intravenous immunoglobulins and time to normalisation. J Infect. 2018;76:206–210. doi: 10.1016/j.jinf.2017.10.017. [DOI] [PubMed] [Google Scholar]
- 31.Lo Cascio G, Koncan R, Stringari G, et al. Interference of confounding factors on the use of (1,3)-beta-d-glucan in the diagnosis of invasive candidiasis in the intensive care unit. Eur J Clin Microbiol Infect Dis. 2015;34:357–365. doi: 10.1007/s10096-014-2239-z. [DOI] [PubMed] [Google Scholar]
- 32.Szyszkowitz A, Zurl C, Herzeg A, et al. Serum 1,3-beta-d-glucan values during and after laparoscopic and open intestinal surgery. Open Forum Infect Dis. 2018;5:ofy296. doi: 10.1093/ofid/ofy296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ikemura K, Ikegami K, Shimazu T, Yoshioka T, Sugimoto T. False-positive result in Limulus test caused by Limulus amebocyte lysate-reactive material in immunoglobulin products. J Clin Microbiol. 1989;27:1965–1968. doi: 10.1128/jcm.27.9.1965-1968.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Miura NN, Ohno N, Aketagawa J, Tamura H, Tanaka S, Yadomae T. Blood clearance of (1 → 3)-beta-d-glucan in MRL lpr/lpr mice. FEMS Immunol Med Microbiol. 1996;13:51–57. doi: 10.1111/j.1574-695X.1996.tb00215.x. [DOI] [PubMed] [Google Scholar]
- 35.White SK, Schmidt RL, Walker BS, Hanson KE. (1→3)-β-d-glucan testing for the detection of invasive fungal infections in immunocompromised or critically ill people. Cochrane Database Syst Rev. 2020;7:CD009833. doi: 10.1002/14651858.CD009833.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Otto GP, Ludewig K, Jacobsen ID, Schaarschmidt B, Hube B, Bauer M. Limitation of (1 → 3)-β-d-Glucan monitoring in major elective surgery involving cardiopulmonary bypass. Crit Care. 2013;17:437. doi: 10.1186/cc12718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mohr JF, Sims C, Paetznick V, et al. Prospective survey of (1 → 3)-beta-D-glucan and its relationship to invasive candidiasis in the surgical intensive care unit setting. J Clin Microbiol. 2011;49:58–61. doi: 10.1128/JCM.01240-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Martín-Mazuelos E, Loza A, Castro C, et al. β-d-Glucan and Candida albicans germ tube antibody in ICU patients with invasive candidiasis. Intensive Care Med. 2015;41:1424–1432. doi: 10.1007/s00134-015-3922-y. [DOI] [PubMed] [Google Scholar]
- 39.Tissot F, Lamoth F, Hauser PM, et al. β-Glucan antigenemia anticipates diagnosis of blood culture-negative intraabdominal candidiasis. Am J Respir Crit Care Med. 2013;188:1100–1109. doi: 10.1164/rccm.201211-2069OC. [DOI] [PubMed] [Google Scholar]
- 40.Posteraro B, Tumbarello M, De Pascale G, et al. (1,3)-β-d-Glucan-based antifungal treatment in critically ill adults at high risk of candidaemia: an observational study. J Antimicrob Chemother. 2016;71:2262–2269. doi: 10.1093/jac/dkw112. [DOI] [PubMed] [Google Scholar]
- 41.León C, Ruiz-Santana S, Saavedra P, et al. Contribution of Candida biomarkers and DNA detection for the diagnosis of invasive candidiasis in ICU patients with severe abdominal conditions. Crit Care. 2016;20:149. doi: 10.1186/s13054-016-1324-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dupuis C, Le Bihan C, Maubon D, et al. Performance of repeated measures of (1–3)-β-d-glucan, mannan antigen, and antimannan antibodies for the diagnosis of invasive candidiasis in ICU patients: a preplanned ancillary analysis of the EMPIRICUS randomized clinical trial. Open Forum Infect Dis. 2021;8:ofab080. doi: 10.1093/ofid/ofab080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Klingspor L, Tortorano AM, Peman J, et al. Invasive Candida infections in surgical patients in intensive care units: a prospective, multicentre survey initiated by the European Confederation of Medical Mycology (ECMM) (2006–2008) Clin Microbiol Infect. 2015;21:87.e1–87.e10. doi: 10.1016/j.cmi.2014.08.011. [DOI] [PubMed] [Google Scholar]
- 44.Alenazy H, Alghamdi A, Pinto R, Daneman N. Candida colonization as a predictor of invasive candidiasis in non-neutropenic ICU patients with sepsis: a systematic review and meta-analysis. Int J Infect Dis. 2021;102:357–362. doi: 10.1016/j.ijid.2020.10.092. [DOI] [PubMed] [Google Scholar]
- 45.León C, Ruiz-Santana S, Saavedra P, et al. Usefulness of the “Candida score” for discriminating between Candida colonization and invasive candidiasis in non-neutropenic critically ill patients: a prospective multicenter study. Crit Care Med. 2009;37:1624–1633. doi: 10.1097/CCM.0b013e31819daa14. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.