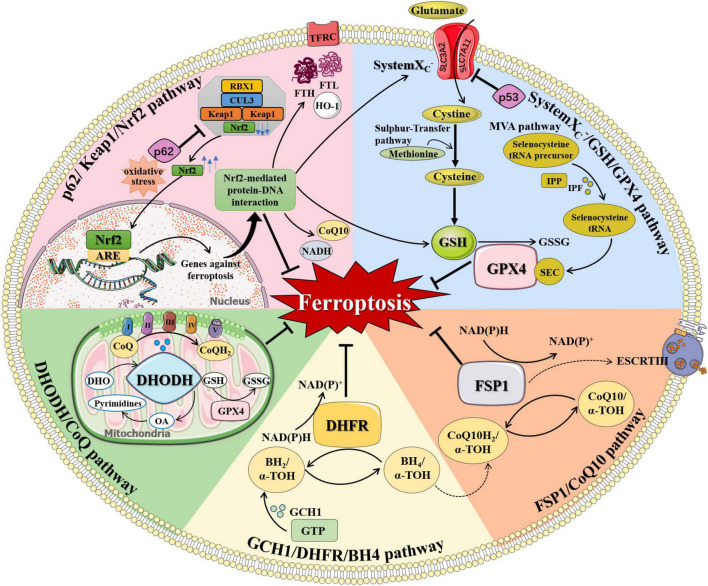
FIGURE 2.
The regulation of ferroptosis. Multiple pathways inhibit ferroptosis by resisting oxidative distress and inhibiting lipid peroxidation, including systemXC–/GSH/GPX4 pathway, FSP1/CoQ10 pathway, GCH1/DHFR/BH4 pathway, DHODH/CoQ pathway, and p62/Keap1/Nrf2 pathway. (1) systemXC–/GSH/GPX4 pathway: systemXC– consists of SLC7A11 and SLC3A2 and is capable to exchange glutamate and cystine equally. Cystine is obtained from methionine through the Sulfur-transfer pathway and then converted to cysteine intracellularly to generate GSH. Selenoprotein GPX4 detects GSH, leading to the reduction of lipid hydroperoxides to lipid alcohols and the simultaneous oxidization of two GSH into oxidized GSSG. IPP, produced by the MVA pathway, transfers isopentyl groups to isopentyl transferases-catalyzed Sec-tRNA precursors which mediate the maturation of Sec-tRNAs responsible for Sec insertion into GPX4. (2) FSP1/CoQ10 pathway: FSP1 converts CoQ10 to CoQ10H2 using NAD(P)H, which quenches lipid free radicals generated by lipid peroxidation. Membrane-associated protein complex ESCRT-III regulates membrane regeneration through membrane germination and cleavage. FSP1 transported in the plasma membrane can resist ferroptosis by enrolling ESCRT-III to activate the membrane restore mechanism. (3) GCH1/DHFR/BH4 pathway: BH4 is a potent free radical scavenger that exerts antioxidant effects in cells. The interconversion between the oxidation and reduction forms of BH4 is controlled by two enzymes, GCH1 and DHFR. The synthesis of BH4 by GCH1 expression selectively prevents PUFA-PL depletion-induced membrane lipid remodeling. DHFR is an essential enzyme for BH4 regeneration, and its inhibition may synergize with GPX4 inhibitors to induce ferroptosis. BH4 can also quench ROS by promoting the synthesis of CoQ10. (4) DHODH/CoQ pathway: DHODH is an enzyme present on the inner surface of mitochondria that catalyzes substrates DHO to produce OA. DHODH cooperates with mitochondrial GPX4 to regulate mitochondrial ferroptosis by reducing CoQ to CoQH2 on the mitochondrial intima independently of the cytoplasmic GPX4 or FSP1 pathways. (5) p62/Keap1/Nrf2 pathway: Under oxidative distress conditions, p62 prevents the degradation of Nrf2 in the Keap1-CUL3-RBX1 E3 ubiquitin ligase complex. Nrf2 undergoes nuclear translocation and binds to ARE to initiate transcription of multiple cytoprotective genes against ferroptosis. Nrf2-mediated protein-DNA interaction regulates the expression of FTH, FTL, FPN, TfR, HO-1, and so on for controlling cellular iron metabolism, promoting SLC7A11 expression, and increasing the production of NADPH, GSH, and CoQ10 to enhance the antioxidant capacity of cells. α-TOH, α-tocopherol; ARE, antioxidant response element; BH2, dihydrobiopterin; BH4, tetrahydrobiopterin; CoQ, coenzyme Q; CoQ10, coenzyme Q10; CoQH2, reduced coenzyme Q; CUL3, cullin 3; DHFR, dihydrofolate reductase; DHO, dihydroorotate acid; DHODH, dihydroorotate dehydrogenase; ESCRT-III, endosome sorting complex; FTH, ferritin heavy chain; FTL, ferritin light chain; FSP1, ferroptosis suppressor protein 1; GCH1, GTP cyclohydrolase-1; GPX4, glutathione peroxidase 4; GSH, glutathione; GSSG, oxidized glutathione; GTP, guanosine triphosphate; HO-1, heme oxygenase 1; IPF, isopentenyl transferase; IPP, isopentenyl pyrophosphate; Keap1, KELCH-ECH-associated protein 1; MVA pathway, mevalonate Regulation pathway; NADH, reduced nicotinamide adenine dinucleotide; NAD(P)+, oxidized nicotinamide adenine dinucleotide (phosphate); NAD(P)H, reduced nicotinamide adenine dinucleotide (phosphate); Nrf2, nuclear factor 2-related erythroid factor 2; OA, orotate; RBX1, RING-box protein 1; Sec, selenocysteine; SLC3A2, solute carrier family 3 member 2; SLC7A11, solute carrier family 7 member 11; TFRC, transferrin receptor.