Abstract
Background and Aims
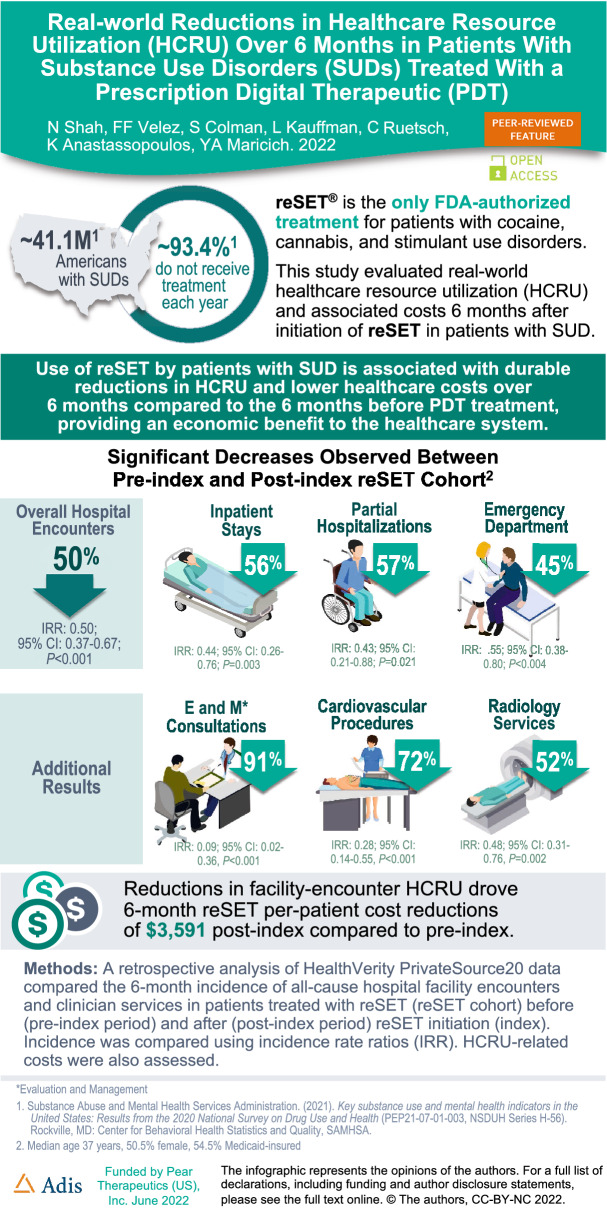
Substance use disorders (SUDs) affect approximately 40.3 million people in the USA, yet only approximately 19% receive evidence-based treatment each year. reSET® is a prescription digital therapeutic (PDT) and the only FDA-authorized treatment for patients with cocaine, cannabis, and stimulant use disorders. This study evaluated real-world healthcare resource utilization (HCRU) and associated costs 6 months after initiation of reSET in patients with SUD.
Methods
A retrospective analysis of HealthVerity PrivateSource20 data compared the 6-month incidence of all-cause hospital facility encounters and clinician services in patients treated with reSET (re-SET cohort) before (pre-index period) and after (post-index period) reSET initiation (index). Incidence was compared using incidence rate ratios (IRR). HCRU-related costs were also assessed.
Results
The sample included 101 patients (median age 37 years, 50.5% female, 54.5% Medicaid-insured). A statistically significant decrease of 50% was observed in overall hospital encounters from pre-index to post-index (IRR 0.50; 95% CI 0.37–0.67; P < 0.001), which included inpatient stays (56% decrease; IRR 0.44; 95% CI 0.26–0.76; P = 0.003), partial hospitalizations (57% decrease; IRR 0.43; 95% CI 0.21–0.88; P = 0.021), and emergency department visits (45% decrease; IRR 0.55; 95% CI 0.38–0.80; P < 0.004). Additionally, some clinician services declined significantly including pathology and laboratory services: other (54% decrease; IRR 0.46; 95% CI 0.28–0.76; P = 0.003); pathology and laboratory services: drug assays prior to opioid medication prescription (37% decrease; IRR 0.63; 95% CI 0.41–0.96; P = 0.031); and alcohol and drug abuse: medication services (46% decrease; IRR 0.54; 95% CI 0.41–0.70; P < 0.001). Reductions in facility-encounters drove 6-month reSET per-patient cost reductions of $3591 post-index compared to pre-index.
Conclusions
Use of reSET by patients with SUD is associated with durable reductions in HCRU and lower healthcare costs over 6 months compared to the 6 months before PDT treatment, after adjusting for covariates, providing an economic benefit to the healthcare system.
Graphical abstract
Supplementary Information
The online version contains supplementary material available at 10.1007/s12325-022-02215-0.
Keywords: Community reinforcement approach, Contingency management, Healthcare resource utilization, Digital therapeutic, DTx, Prescription digital therapeutic, PDT, reSET, Substance use disorders, SUD
Key Summary Points
| Why carry out this study? |
| Substance use disorders (SUDs) place a heavy cost burden on healthcare systems and society at large. |
| Many barriers to effective treatment of SUDs may be overcome with prescription digital therapeutics (PDTs) delivering evidence-based, FDA-approved treatments to patients via mobile devices. |
| This study evaluated the real-world 6-month impact on healthcare resource utilization (HCRU) in 101 patients with SUDs treated with the reSET® PDT. |
| What was learned from this study? |
| Comparing the 6 months before treatment to the 6 months after treatment with the PDT revealed significant decreases in overall hospital encounters, inpatient stays, partial hospitalizations, and emergency department visits. |
| Reductions in facility-encounter HCRU drove 6-month per-patient cost reductions of $3591 post-treatment compared to pre-treatment. |
Digital Features
This article is published with digital features, including an infographic to facilitate understanding of the article. To view digital features for this article go to 10.6084/m9.figshare.19950266.
Introduction
Substance use disorders (SUDs) are highly heterogeneous conditions [1] affecting approximately 40.3 million people in the USA, and yet the most recently available data show that only about 6.5% of these people ever receive SUD treatment [2]. These challenges have been exacerbated during the COVID-19 pandemic [2]. According to the Centers for Disease Control and Prevention, as of June 2020, 13% of US adults reported starting or increasing substance use as a way of coping with stress or emotions related to COVID-19 [3]. The pandemic has also added an additional layer of challenge for treatment service providers as many have had to quickly adopt an online format to continue operations. Furthermore, although behavioral and psychosocial treatments for SUD have long existed [4], most patients do not receive these therapies because of a lack of specialty facilities and/or trained clinicians or geographical barriers in rural communities [5–7].
A wider use of behavioral therapies is also impeded by inconsistent delivery, quality, and fidelity across healthcare providers [8], and high attrition rates among providers [9]. It has been suggested that virtual support programs can assist patients in recovery or reduce misuse, especially when access to physicians can be challenging [10–12]. Being forced into isolation, quarantines, curfews, and shutdowns have limited access to in-person supportive programs and have heightened the risk for increased alcohol consumption and drug use, especially among predisposed individuals [13, 14].
The many challenges posed by SUDs fall disproportionately on underserved populations such as older adults, racial/ethnic minorities, rural populations, military veterans, low-income individuals, and sexual and gender minorities [15]. SUDs also place a heavy cost burden on healthcare systems and society at large. A 2021 study found that the total annual SUD medical cost in hospitals was $13.2 billion with annual costs varying by substance type (e.g., $4 million for inhalant-related disorders and $7.6 billion for alcohol-related disorders) [16].
Goals of treatment for various SUDs include harm reduction and early intervention with cognitive behavioral therapy (CBT) and/or contingency management (CM) [17]. Harm reduction with CBT can significantly reduce use of substances and the impact of the complex problems associated with use [18, 19]. For example, reduced alcohol consumption is associated with physical and psychological benefits [20], and there is strong empirical support for early intervention (i.e., treatment of lower-severity AUD and those in earlier stages of addiction) using harm reduction strategies [18].
Studies have consistently demonstrated that CM can support engagement and abstinence in patients with SUDs, particularly those with use disorders related to cannabis, cocaine, and stimulants [21] with CM showing the highest individual effect size in a meta-analysis of techniques for treating SUDs [22].
Digital therapeutic approaches have the potential to deliver high-fidelity, evidence-based mechanisms of action, to reach broad patient populations across a variety of diseases, and to tailor treatment to patients’ needs, stages of recovery, and clinical trajectories. They can be delivered in a patient-centric manner across diverse care settings and can address variables that constitute social determinants of health [23]. Prescription digital therapeutics (PDTs) are software-based disease treatments evaluated for safety and effectiveness in randomized controlled trials (RCTs) and authorized by the US Food and Drug Administration (FDA). Prescribed by treating physicians, and delivered on mobile devices, PDTs can expand access to evidence-based behavioral therapies across a variety of diseases, including SUDs.
The reSET® PDT is a 12-week therapeutic combining CBT based on the Community Reinforcement Approach (CRA) [24], a CM system providing motivational incentives for lesson completion and abstinence, and fluency training to reinforce concept mastery [25]. It is the only FDA-authorized treatment for adult patients with cocaine, cannabis, and stimulant use disorders.
The clinical effectiveness of reSET was evaluated in a number of RCTs involving approximately 1500 patients with SUDs [26–32], and a real-world study in 602 patients [33]. These studies demonstrated improved rates of abstinence and treatment retention among patients receiving the digital therapy as an adjunct to reduced treatment-as-usual (TAU) compared to those who received TAU alone.
Real-world data acquired in the context of healthcare delivery and in the absence of stringent research constraints provide a complementary evaluation of therapeutic performance, including measures of patient engagement and clinical outcomes [34, 35]. To date, however, no data have yet been published about the impact of reSET use on healthcare resource utilization (HCRU) and costs of care.
The objective of this study was to evaluate the real-world HCRU impact in an early cohort of commercially insured patients treated with the reSET PDT.
Methods
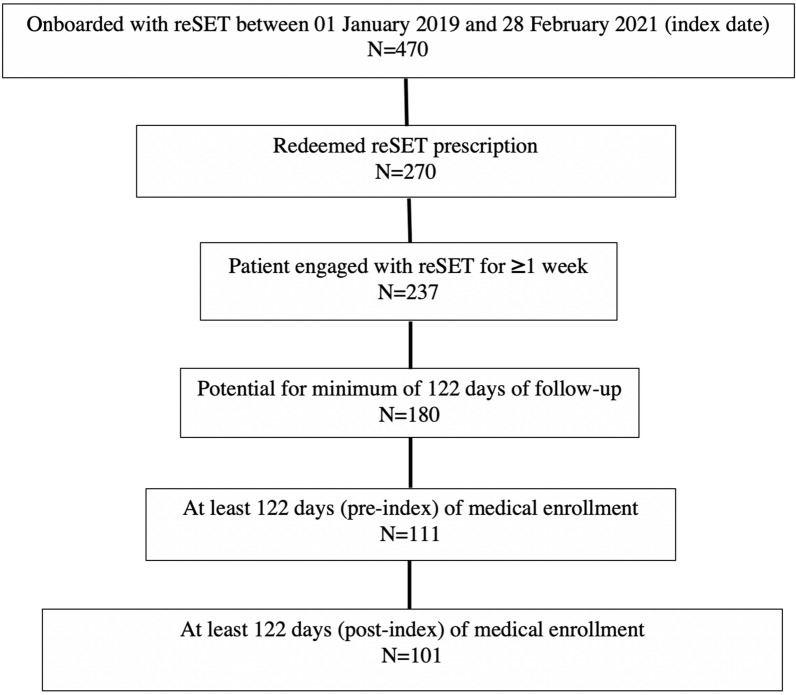
A 6-month observational retrospective analysis of HealthVerity PrivateSource20 closed-claims data (January 1, 2018 through February 28, 2021) was conducted in adults in the USA diagnosed with SUD and prescribed reSET. Patients who filled their reSET prescription (index date) and engaged with the therapeutic for longer than 1 week were included in the reSET cohort (Fig. 1). All patients were required to have at least 4 months of enrollment in the pre-index and post-index periods.
Fig. 1.
CONSORT diagram for reSET 6-month pre/post analyses
All patients had been deemed by their prescribing clinician as being appropriate for the PDT. The incidence of HCRU was compared between the 6-month pre-index period and the 6-month post-index period.
A scenario analysis of the cost impact of changes in facility and clinical service encounters was conducted using published facility costs for patients with OUD: $19,023 for inpatient (IP) stays [36], $124,419 for intensive care unit (ICU) stays [24], $1969 for emergency department (ED) visits [16], and 2020 Medicare reimbursement rates for remaining facility and clinician services, as has been performed in previous analyses [37]. These costs were multiplied by the number of events in each category and averaged by patient to derive the net cost reductions/increases.
Data Sources and Statistical Analyses
The PrivateSource20 data source includes closed medical claims for approximately 70 million commercial, 60 million Medicaid, and 15 million Medicare Advantage enrollees across 150 payers since 2015. All US census regions and states, Washington DC, and Puerto Rico are represented. The data include enrollment history and healthcare claims for both pharmacy and medical benefits from all settings of care. Medical claims include diagnoses and procedures for exact dates of service. Pharmacy claims include prescribed medications, prescription date, days’ supply, and quantity dispensed.
The analysis examined the differences in the incidence of HCRU encounters between the pre-index and post-index periods. The incidence for each HCRU encounter type was calculated using a repeated measures (pre and post) negative binomial model. An incidence rate ratio (IRR) was calculated as the incidence in the post-index period relative to the incidence in the pre-index period, and was used to compare the pre-index and post-index incidence (e.g., IRR < 1 indicates lower HCRU in the post-index period compared to the pre-index period). The 95% confidence intervals for the incidences and the IRR were also calculated along with the IRR P value. Analyses were performed using SAS statistical software (SAS Institute Inc. Cary, North Carolina, USA).
This study received a waiver of authorization for the use and disclosure of protected health information (PHI) and a determination of exempt status under 45 CFR § 46.104(d)(4) from Western Institutional Review Board on October 21, 2021.
PDT Description
The reSET CBT content consists of a series of interactive, on-demand audio, text, and video modules that are sequentially unlocked as patients progress through the program with CM providing motivational incentives for lesson completion and abstinence. Patients are instructed to complete four modules per week starting with the 31 core modules and then, when those are completed, an additional 30 supplemental modules.
Core modules teach basic cognitive behavioral and relapse prevention skills, and provide education about behavioral risk reduction for infections related to sex or shared needles. Supplemental modules target improved psychosocial functioning (e.g., managing relationships, building communication skills, and improving time management) and provide in-depth training on preventing or living with infections. Upon successful completion of each module the patient undergoes fluency training via a quiz and they have a chance to “spin” the digital CM wheel to be eligible for either virtual rewards (e.g., a “thumbs up” icon) or digital gift certificates ranging in value from $5 to $100, with the odds of winning a certificate higher for lower-value certificates. Patient-reported outcomes can be recorded in the PDT, and the clinical care team can enter urine drug screen results, with negative results allowing the patient another CM wheel spin.
Study Measures
This study evaluated patient demographic characteristics including age, sex, geographic region, and payer type. Claims in the pre-index and post-index period were identified as facility claims or clinician service claims in order to characterize patients’ HCRU. All-cause hospital facility encounters were evaluated overall and individually for inpatient (IP) stays, emergency department (ED) visits, partial hospitalizations (PH), and hospital outpatient department (HOPD) surgical visits. Among the inpatient stays, intensive care unit (ICU) stays were examined. Clinician services included categories of Current Procedure Terminology (CPT) codes identified from clinician claims such as evaluation and management (E&M), specific medical services (e.g., cardiovascular, psychiatry, neurology), pathology and laboratory, and rehabilitative services. Costs associated with facility encounters were evaluated.
Results
The study included 101 patients who were prescribed and used reSET with a median age of 37 years, 50.5% female, 54.5% Medicaid-insured, and 37.6% commercially insured (Table 1). Approximately 71.4% of patients had an alcohol-related disorder, 41.6% had a nicotine-related disorder, 38% had an opioid-related disorder, 33.7% had a cannabis-related disorder, 29.7% had a cocaine-related disorder, and 10.9% had a disorder related to a stimulant other than cocaine. (Percentages do not add to 100% because patients often have multiple substance disorders.) Approximately one-quarter of patients (N = 24) were prescribed naltrexone in either the pre-index or post-index periods.
Table 1.
Patient demographics and characteristics
| Demographic/characteristic | reSET cohort (N = 101) |
|---|---|
| Median age | 37 |
| Female sex | 50.5% |
| Mean Charlson comorbidity score | 0.802 |
| Payer, n (%) | |
| Commercial | 38 (37.6%) |
| Medicaid | 55 (54.5%) |
| Medicare advantage | 3 (3.0%) |
| Unknown | 5 (5.0%) |
| Census region, n (%) | |
| Middle Atlantic | 41 (40.6%) |
| East North Central | 17 (16.8%) |
| South Atlantic | 12 (11.9%) |
| Other | 31 (30.7%) |
A statistically significant decrease of 50% was observed in overall hospital facility encounters from the pre-index period (incidence = 1.326) to the post-index period (incidence = 0.661) (IRR 0.50; 95% CI 0.37–0.67; P < 0.001) (Table 2). Statistically significant decreases were observed for inpatient stays (56% decrease; IRR 0.44; 95% CI 0.26–0.76; P = 0.003), PHs (57% decrease; IRR 0.43; 95% CI 0.21–0.88; P = 0.021), and ED visits (not admitted) (45% decrease; IRR 0.55; 95% CI 0.38–0.80; P < 0.002). Decreases in HOPD surgical visits were not significant (50% decrease; IRR 0.50; 95% CI 0.12–2.04; P < 0.335).
Table 2.
reSET cohort pre/post analysis of incidence of hospital facility services over 6 months
| Resource | Pre-index period (N = 101) | Post-index period (N = 101) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| n (%) | Incidence | 95% CI | n (%) | Incidence | 95% CI | IRR | 95% CI | p value | |
| Unique hospital encounters | 46 (45.5%) | 1.326 | (0.974, 1.805) | 31 (30.7%) | 0.661 | (0.456, 0.959) | 0.50 | (0.37, 0.67) | < 0.001 |
| Inpatient stays | 22 (21.8%) | 0.320 | (0.209, 0.488) | 12 (11.9%) | 0.142 | (0.081, 0.248) | 0.44 | (0.26, 0.76) | 0.003 |
| ICU stays | 0 (0.0%) | 0.000 | (0.000, 0.030) | 2 (2.0%) | 0.020 | (0.005, 0.081) | NA | NA | NA |
| Readmissions | 6 (5.9%) | 0.080 | (0.035, 0.182) | 1 (1.0%) | 0.010 | (0.001, 0.071) | 0.13 | (0.02, 1.07) | 0.058 |
| Partial hospitalizations | 9 (8.9%) | 0.278 | (0.130, 0.595) | 6 (5.9%) | 0.120 | (0.053, 0.269) | 0.43 | (0.21, 0.88) | 0.021 |
| ED visits—not admitted | 35 (34.7%) | 0.710 | (0.497, 1.013) | 22 (21.8%) | 0.391 | (0.244, 0.627) | 0.55 | (0.38, 0.80) | 0.002 |
| HOPD visits | 2 (2.0%) | 0.020 | (0.005, 0.079) | 1 (1.0%) | 0.010 | (0.001, 0.072) | 0.50 | (0.12, 2.04) | 0.335 |
n number of patients with at least 1 encounter, CI confidence interval, ED emergency department, HOPD hospital outpatient department, IRR incidence rate ratio, NA not applicable
Significant changes were observed in the use of a number of clinician services. The top four service categories with the largest changes in terms of number of events in the observational periods included pathology and laboratory services: other (54% decrease; IRR 0.46; 95% CI 0.28–0.76; P = 0.003); pathology and laboratory services: drug assays prior to opioid medication prescription (37% decrease; IRR 0.63; 95% CI 0.41–0.96; P = 0.031); alcohol and drug abuse: medication services (46% decrease; IRR 0.54; 95% CI 0.41–0.70; P < 0.001); and alcohol and drug abuse: treatment program (11% decrease; IRR 0.89; 95% CI 0.61–1.30; P = 0.552) (see supplementary Table 1 for a complete listing of clinician and facility services data).
Reductions in hospital facility HCRU drove 6-month, per-patient net cost reductions of $3591 in the post-index period.
Discussion
Acute healthcare utilization attributed to SUD is continuing to rise, particularly among patients with stimulant use disorder [38, 39], and in minority populations [2]. This is the first real-world evaluation to describe the economic and clinical effectiveness of the only SUD treatment intervention currently FDA-authorized for patients with cocaine, cannabis, and stimulant use disorders. Expansion of the Affordable Care Act’s SUD services to Medicaid recipients as well as to young adults between the ages of 18 and 23 may have resulted in an increase in treatments for these patients [40, 41], but the efficacy of these treatments and the net benefit have yet to be determined. No other treatment intervention for SUD to our knowledge has exhibited real-world effectiveness and net monetary benefit to the health system.
This study showed that within-patient hospital encounters dropped by 50%, and ED visits by 45% in the 6-month period after treatment with reSET compared to a similar baseline period. Patients with SUDs often seek care in the ED, and overuse of services in this setting unnecessarily burdens the healthcare system. The situation is complicated for patients with ED presentations related to cocaine and psychostimulants because such visits may not be identified as related to drug toxicity/withdrawal and, instead, be described by the interventions used, such as acute cardiopulmonary services or psychiatric interventions [38].
The reductions in service usage we observed are particularly significant in the context of reported increases in service usage during the COVID-19 pandemic among patients with SUDs. Although hospitalization rates for certain disease states declined during the pandemic (e.g., acute cardiovascular disease [42], and stroke/TIAs [43]), several published data sets and analyses show that among patients with existing SUDs the rate of ED visits and hospitalizations increased during the pandemic. This may partially be due to an increased susceptibility to COVID among people with SUDs. For example, a 2022 study found that people with SUDs or alcohol use disorders have a greater probability of being hospitalized for COVID-19 infections compared to the general population [44]. Regardless of the cause, however, a 2021 report by Holland et al. in JAMA Psychiatry found that, compared to pre-pandemic rates in 2019, rates of ED visits related to all-drug overdoses rose from 12,891 to 14,959 per 100,000 ED visits, and the rates of visits related to opioid-related overdoses rose from 3940 to 5075 per 100,000 ED visits [45].
We were unable to perform subgroup analyses by gender, race, and insurance status because of the small sample sizes. Evidence in the literature clearly reveals a significant unmet need for SUD treatments among racial minorities, reproductive-age women, and veterans, all of whom are disproportionately vulnerable to SUD and its deleterious effects [46–48]. Future investigations should focus on these populations.
Limitations
Potential limitations exist that are common with all healthcare claims-based analyses. For instance, mortality cannot be assessed using claims data and it is possible that some patients may have died in the post-index period. Another limitation of claims data is the potential for data entry errors, as well as the absence of medical history context (which can affect the interpretability of the observed trends). Cost calculations are limited to estimates derived from the published literature and therefore they may not be representative of actual costs, which will vary across different healthcare plans. A limitation of a pre/post design is that the potential impact of secular trends is limited by the period (e.g., 6 months) over which incidence is assessed and compared pre/post. It is important to note that observational research complements the evidence from randomized controlled trials on which FDA authorization decisions are made because real-world, post-authorization studies provide evidence from all-comer populations being treated in a wide range of locations and settings, which is very different from the selected populations being treated in highly controlled environments typical of clinical trials.
Conclusions
This real-world evaluation in diverse patients with SUD treated with the reSET PDT showed significant decreases in unique hospital encounters and ED visits 6 months after initiation of the PDT compared to the 6 months before treatment. The observed per-patient cost reduction of $3591 in the pre-post analysis suggests that treating patients with reSET may lower overall costs of care, which may be relevant to payors at all levels of the healthcare system.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors thank Stephen Braun and Rowan Mahon for editorial assistance in the preparation of this manuscript.
Funding
This study and journal’s Rapid Service Fee were funded entirely by Pear Therapeutics (US), Inc.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by Samuel Colman, Laura Kauffman, Charles Ruetsch, and Kathryn Anastassopoulos. The first draft of the manuscript was written by Neel Shah, and all authors commented on subsequent versions of the manuscript. All authors read and approved the final manuscript.
Disclosures
Neel Shah, Fulton Velez, and Yuri Maricich are employees of Pear Therapeutics (US), Inc. Samuel Colman, Laura Kauffman, and Kathryn Anastassopoulos are employees Market Access Consulting, Labcorp Drug Development, which participated in this study under contract with Pear Therapeutics (US), Inc. Charles Ruetsch is an employee of Health Analytics, which participated in this study under contract with Pear Therapeutics (US), Inc.
Compliance with Ethics Guidelines
This study received a waiver of authorization for the use and disclosure of protected health information (PHI) and a determination of exempt status under 45 CFR § 46.104(d)(4) from Western Institutional Review Board on October 21, 2021.
References
- 1.Carroll KM. The profound heterogeneity of substance use disorders: implications for treatment development. Curr Dir in Psychol Sci. 2021;30(4):358–364. doi: 10.1177/09637214211026984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Substance Abuse and Mental Health Services Administration. Key substance use and mental health indicators in the United States: results from the 2020 National Survey on Drug Use and Health (HHS Publication No. PEP21-07-01-003, NSDUH Series H-56). 2020. https://www.samhsa.gov/data/. Accessed 28 Oct 2021.
- 3.Centers for Disease Control & Prevention. Mental health, substance use, and suicidal ideation during the COVID-19 pandemic. https://www.cdc.gov/mmwr/volumes/69/wr/mm6932a1.htm. Accessed 9 Mar 2022.
- 4.Miller SC, Fielin DA, Rosenthal RN, Saitz R. The ASAM principles of addiction medicine. 6th ed. Philadelphia: Wolters Kluwer; 2018.
- 5.Moore BA, Fazzino T, Garnet B, Cutter CJ, Barry DT. Computer-based interventions for drug use disorders: a systematic review. J Subst Abuse Treat. 2011;40(3):215–223. doi: 10.1016/j.jsat.2010.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kiluk BD, Sugarman DE, Nich C, et al. A methodological analysis of randomized clinical trials of computer-assisted therapies for psychiatric disorders: toward improved standards for an emerging field. Am J Psychiatry. 2011;168(8):790–799. doi: 10.1176/appi.ajp.2011.10101443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marsch LA, Carroll KM, Kiluk BD. Technology-based interventions for the treatment and recovery management of substance use disorders: a JSAT special issue. J Subst Abuse Treat. 2014;46(1):1–4. doi: 10.1016/j.jsat.2013.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Institute of Medicine . Improving the quality of health care for mental and substance-use conditions. Washington, DC: The National Academies Press; 2006. p. 528. [PubMed] [Google Scholar]
- 9.Lappan SN, Brown AW, Hendricks PS. Dropout rates of in-person psychosocial substance use disorder treatments: a systematic review and meta-analysis. Addiction. 2020;115(2):201–217. doi: 10.1111/add.14793. [DOI] [PubMed] [Google Scholar]
- 10.Bergman BG, Kelly JF, Fava M, Eden EA. Online recovery support meetings can help mitigate the public health consequences of COVID-19 for individuals with substance use disorder. Addict Behav. 2021;113:106661. doi: 10.1016/j.addbeh.2020.106661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Spagnolo PA, Montemitro C, Leggio L. New challenges in addiction medicine: COVID-19 infection in patients with alcohol and substance use disorders—the perfect storm. Am J Psychiatry. 2020;177(9):805–807. doi: 10.1176/appi.ajp.2020.20040417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Molfenter T, Heitkamp T, Murphy AA, Tapscott S, Behlman S, Cody OJ. Use of telehealth in mental health (MH) services during and after COVID-19. Community Ment Health J. 2021;57(7):1244–1251. doi: 10.1007/s10597-021-00861-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marsden J, Darke S, Hall W, et al. Mitigating and learning from the impact of COVID-19 infection on addictive disorders. Addiction. 2020;115(6):1007–1010. doi: 10.1111/add.15080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Horigian VE, Schmidt RD, Feaster DJ. Loneliness, mental health, and substance use among US young adults during COVID-19. J Psychoactive Drugs. 2021;53(1):1–9. doi: 10.1080/02791072.2020.1836435. [DOI] [PubMed] [Google Scholar]
- 15.National Academies of Sciences Engineering and Medicine. Communities in action: pathways to health equity. National Academies Press; 2017. https://www.nap.edu/catalog/24624/communities-in-action-pathways-to-health-equity. Accessed 8 Mar 2022. [PubMed]
- 16.Peterson C, Li M, Xu L, Mikosz CA, Luo F. Assessment of annual cost of substance use disorder in US hospitals. JAMA Netw Open. 2021;4(3):e210242. doi: 10.1001/jamanetworkopen.2021.0242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Paquette CE, Daughters SB, Witkiewitz K. Expanding the continuum of substance use disorder treatment: nonabstinence approaches. Clin Psychol Rev. 2022;91:102110. doi: 10.1016/j.cpr.2021.102110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Charlet K, Heinz A. Harm reduction-a systematic review on effects of alcohol reduction on physical and mental symptoms. Addict Biol. 2017;22(5):1119–1159. doi: 10.1111/adb.12414. [DOI] [PubMed] [Google Scholar]
- 19.Marlatt GA, Witkiewitz K. Harm reduction approaches to alcohol use: health promotion, prevention, and treatment. Addict Behav. 2002;27(6):867–886. doi: 10.1016/S0306-4603(02)00294-0. [DOI] [PubMed] [Google Scholar]
- 20.Witkiewitz K, Kranzler HR, Hallgren KA, et al. Stability of drinking reductions and long-term functioning among patients with alcohol use disorder. J Gen Intern Med. 2021;36(2):404–412. doi: 10.1007/s11606-020-06331-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Benishek LA, Dugosh KL, Kirby KC, et al. Prize-based contingency management for the treatment of substance abusers: a meta-analysis. Addiction. 2014;109(9):1426–1436. doi: 10.1111/add.12589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Prendergast M, Podus D, Finney J, Greenwell L, Roll J. Contingency management for treatment of substance use disorders: a meta-analysis. Addiction. 2006;101(11):1546–1560. doi: 10.1111/j.1360-0443.2006.01581.x. [DOI] [PubMed] [Google Scholar]
- 23.Lord SE, Campbell ANC, Brunette MF, et al. Workshop on implementation science and digital therapeutics for behavioral health. JMIR Ment Health. 2021;8(1):e17662. doi: 10.2196/17662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Budney AJ, Higgins ST. Therapy manuals for drug addiction, a community reinforcement plus vouchers approach: treating cocaine addiction. Rockville: National Institute on Drug Abuse; 1998.
- 25.Petry NM, Alessi SM, Olmstead TA, Rash CJ, Zajac K. Contingency management treatment for substance use disorders: how far has it come, and where does it need to go? Psychol Addict Behav. 2017;31(8):897–906. doi: 10.1037/adb0000287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bickel WK, Marsch LA, Buchhalter AR, Badger GJ. Computerized behavior therapy for opioid-dependent outpatients: a randomized controlled trial. Exp Clin Psychopharmacol. 2008;16(2):132–143. doi: 10.1037/1064-1297.16.2.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marsch LA, Guarino H, Acosta M, et al. Web-based behavioral treatment for substance use disorders as a partial replacement of standard methadone maintenance treatment. J Subst Abuse Treat. 2014;46(1):43–51. doi: 10.1016/j.jsat.2013.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Christensen DR, Landes RD, Jackson L, et al. Adding an internet-delivered treatment to an efficacious treatment package for opioid dependence. J Consult Clin Psychol. 2014;82(6):964–972. doi: 10.1037/a0037496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Campbell AN, Nunes EV, Matthews AG, et al. Internet-delivered treatment for substance abuse: a multisite randomized controlled trial. Am J Psychiatry. 2014;171(6):683–690. doi: 10.1176/appi.ajp.2014.13081055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chaple M, Sacks S, McKendrick K, et al. A comparative study of the therapeutic education system for incarcerated substance-abusing offenders. Prison J. 2016;96(3):485–508. doi: 10.1177/0032885516636858. [DOI] [Google Scholar]
- 31.Luderer HF, Campbell ANC, Nunes EV, et al. Engagement patterns with a digital therapeutic for substance use disorders: correlations with abstinence outcomes. J Subst Abuse Treat. 2022;132:108585. doi: 10.1016/j.jsat.2021.108585. [DOI] [PubMed] [Google Scholar]
- 32.Maricich Y, Nunes EV, Campbell ANC, et al. Safety and efficacy of a digital therapeutic for substance use disorder: secondary analysis of data from a NIDA Clinical Trials Network Study. Subst Abuse. 2022;43(1):937–942. doi: 10.1080/08897077.2022.2060425. [DOI] [PubMed] [Google Scholar]
- 33.Xiong X, Braun S, Stitzer M, et al. Evaluation of real-world outcomes associated with use of a prescription digital therapeutic to treat substance use disorders. Am J Addict. 2022. (in press). [DOI] [PMC free article] [PubMed]
- 34.Dreyer NA. Advancing a framework for regulatory use of real-world evidence: when real is reliable. Ther Innov Regul Sci. 2018;52(3):362–368. doi: 10.1177/2168479018763591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Blonde L, Khunti K, Harris SB, Meizinger C, Skolnik NS. Interpretation and impact of real-world clinical data for the practicing clinician. Adv Ther. 2018;35(11):1763–1774. doi: 10.1007/s12325-018-0805-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Arifkhanova A, McCormick Kraus E, Al-Tayyib A, et al. Estimating costs of hospitalizations associated with opioid use disorder or opioid misuse at a large, urban safety-net hospital-Denver, Colorado, 2017. Drug Alcohol Depend. 2021;218:108306. doi: 10.1016/j.drugalcdep.2020.108306. [DOI] [PubMed] [Google Scholar]
- 37.Centers for Medicare and Medicaid Services (CMS). Medicare hospital outpatient prospective payment system addendum A, April 2020 file. 2020. https://www.cms.gov/Medicare/Medicare-Fee-For-Service-Payment/HospitalOutpatientPPS/Addendum-A-and-Addendum-B-Updates. Accessed 28 July 2020.
- 38.Suen LW, Davy-Mendez T, LeSaint KT, Riley ED, Coffin PO. Emergency department visits and trends related to cocaine, psychostimulants, and opioids in the United States, 2008–2018. BMC Emerg Med. 2022;22(1):19. doi: 10.1186/s12873-022-00573-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jones CM, Houry D, Han B, Baldwin G, Vivolo-Kantor A, Compton WM. Methamphetamine use in the United States: epidemiological update and implications for prevention, treatment, and harm reduction. Ann N Y Acad Sci. 2022;1508(1):3–22. doi: 10.1111/nyas.14688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Maclean JC, Saloner B. The effect of public insurance expansions on substance use disorder treatment: evidence from the affordable care act. J Policy Anal Manage. 2019;38(2):366–393. doi: 10.1002/pam.22112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Saloner B, Akosa Antwi Y, Maclean JC, Cook B. Access to health insurance and utilization of substance use disorder treatment: evidence from the affordable care act dependent coverage provision. Health Econ. 2018;27(1):50–75. doi: 10.1002/hec.3482. [DOI] [PubMed] [Google Scholar]
- 42.Kiss P, Carcel C, Hockham C, Peters SAE. The impact of the COVID-19 pandemic on the care and management of patients with acute cardiovascular disease: a systematic review. Eur Heart J Qual Care Clin Outcomes. 2021;7(1):18–27. doi: 10.1093/ehjqcco/qcaa084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mariet AS, Giroud M, Benzenine E, et al. Hospitalizations for stroke in France during the COVID-19 pandemic before, during, and after the national lockdown. Stroke. 2021;52(4):1362–1369. doi: 10.1161/STROKEAHA.120.032312. [DOI] [PubMed] [Google Scholar]
- 44.Pavarin RM, Fabbri C, De Ronchi D. COVID-19 hospitalization rates in individuals with substance or alcohol use disorders. Psychiatry Res. 2022;311:114521. doi: 10.1016/j.psychres.2022.114521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Holland KM, Jones C, Vivolo-Kantor AM, et al. Trends in US emergency department visits for mental health, overdose, and violence outcomes before and during the COVID-19 pandemic. JAMA Psychiat. 2021;78(4):372–379. doi: 10.1001/jamapsychiatry.2020.4402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mulvaney-Day N, DeAngelo D, Chen CN, Cook BL, Alegria M. Unmet need for treatment for substance use disorders across race and ethnicity. Drug Alcohol Depend. 2012;125(Suppl 1):S44–50. doi: 10.1016/j.drugalcdep.2012.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Martin CE, Scialli A, Terplan M. Unmet substance use disorder treatment need among reproductive age women. Drug Alcohol Depend. 2020;206:107679. doi: 10.1016/j.drugalcdep.2019.107679. [DOI] [PubMed] [Google Scholar]
- 48.Golub A, Vazan P, Bennett AS, Liberty HJ. Unmet need for treatment of substance use disorders and serious psychological distress among veterans: a nationwide analysis using the NSDUH. Mil Med. 2013;178(1):107–114. doi: 10.7205/MILMED-D-12-00131. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.