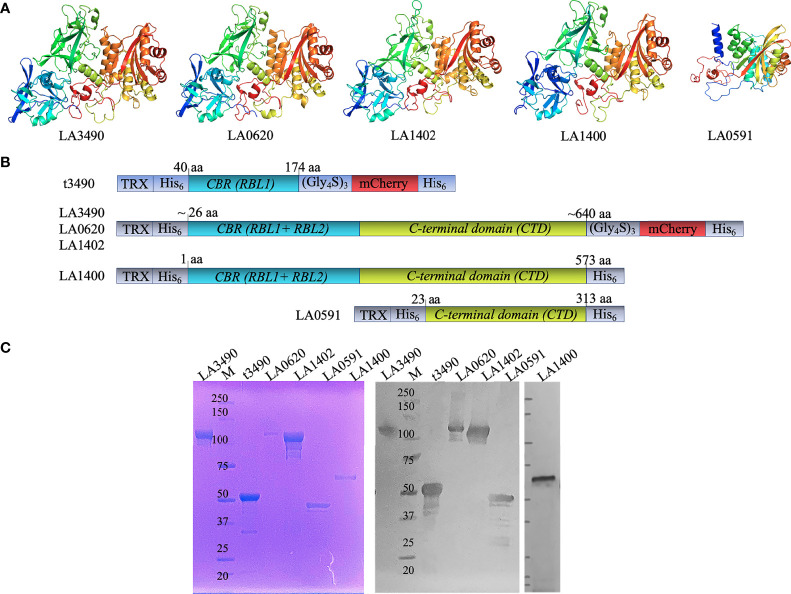
Figure 1.
DeepMind AlphaFold algorithm derived structure, strategy for cloning, purification, and antigenicity of recombinant His-tagged VM proteins. (A) Artificial intelligence-based high-resolution structural modeling of (LA3490, LA0620, LA1402, LA1400 and LA0591) using AlphaFold algorithm [Callaway, E. (2020)]. (B) Schematic diagram depicting the organization of the recombinant mCherry (mC) fusion VM proteins used in the current study; t3490, amino acid positions 40 aa -147 aa (minus signal sequence); LA3490 (19 aa – 639 aa), LA0620 (32 aa – 637 aa), LA1402 (28 aa - 641 aa), LA1400 (1 aa - 573 aa), and LA0591 (23 aa – 313 aa). The clones were designed without signal sequences. LA1400 naturally lack signal sequence. Recombinant fusions include a glycine-serine (Gly4S)3 linker (for flexibility), N-and C-terminal His6 tag (purification), and N-terminal thioredoxin. (C) AKTA purified soluble His-tagged VM proteins (LA3490, t3490, LA0620, LA1402, LA0591, and LA1400) were analyzed by 4 ± 12% SDS-PAGE followed by Coomassie staining. A replicate gel was run for immunoblot analysis. The proteins were transferred to a nitrocellulose membrane and the blot was probed with mouse anti-His monoclonal-ALP conjugate (1:2,000 dilution; Santa Cruz Biotechnology, USA). M represents molecular weight marker.