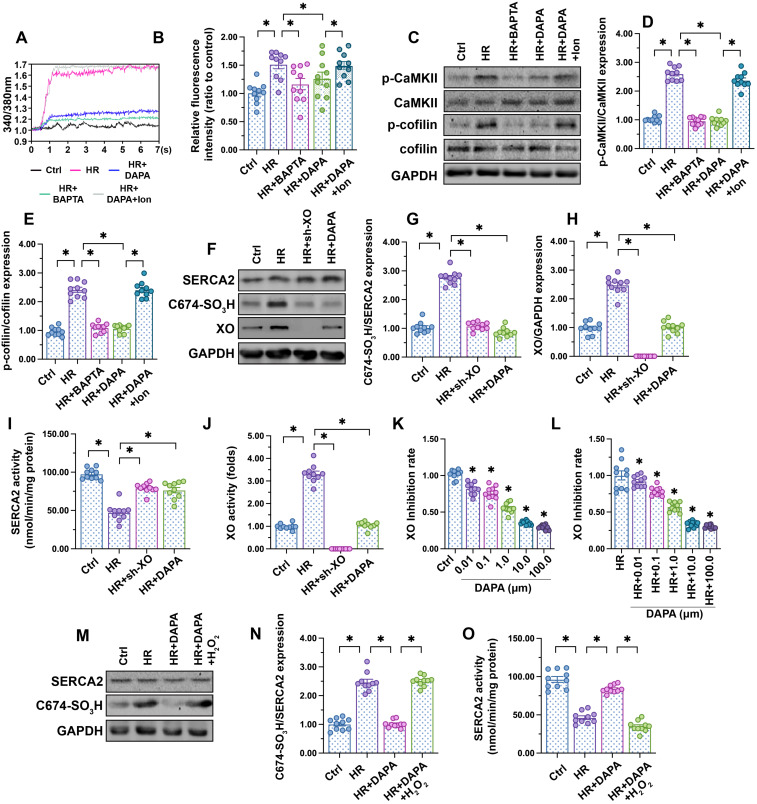
Figure 4.
DAPA inhibits XO-mediated SERCA2 oxidation and normalizes intracellular calcium balance. Human coronary artery endothelial cells (HCAECs) were treated with 45-min hypoxia (H) followed by a 2-h reoxygenation (R) phase to induce H/R injury. DAPA (10 µM) was applied to HCAECs 24 h before H/R. (A, B) HCAECs were incubated on the confocal microscopy in the presence of 1 µM Fura-2AM for 2 minutes while monitoring the fluorescence intensities at 340 nm and 380 nm (excitation). The ratio of the emissions at 340 nm and 380 nm wavelengths is directly related to the amount of intracellular Ca2+. The relative fluorescence intensity was normalized to that of the control group. HCAECs were treated with BAPTA (50 µM) or ionomycin (Ion, 100 µM) for 30 min before H/R to prevent and induce calcium overload, respectively. (C-E) Western blot analysis of p-cofilin and p-CaMKII expression in HCAECs. (F-H) Western blot analysis of SERCA2 oxidation (Cys-674) and XO expression. Lentiviral shRNA targeting XO was transduced into HCAECs and knockout efficiency confirmed by western blotting. (I, J) ELISA was used to analyze changes in SERCA2 and XO activities. (K-L) XO inhibitory activity via in vitro system. *p < 0.05 vs. control group or H/R group. (M-N) Western blot analysis of SERCA2 oxidation (Cys-674). To induce a pro-oxidative microenvironment, HCAECs were treated with 0.3 mM hydrogen peroxide for 6 h before DAPA treatment. (O) ELISA was used to analyze changes in SERCA2 activity. To induce a pro-oxidative microenvironment, HCAECs were treated with 0.3 mM hydrogen peroxide for 6 h before DAPA treatment. Experiments were repeated at least three times and the data are shown as mean ± SEM (n = ten independent cell isolations per group). *p < 0.05.