FP@PdII-Salen-[IM]OH-catalyzed C–C Sonogashira cross-coupling reactiona.
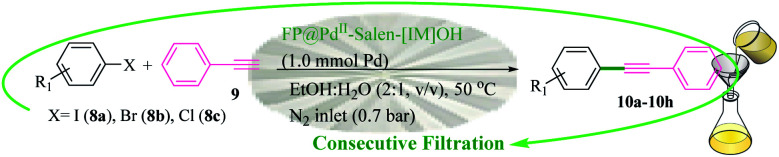
| ||||
|---|---|---|---|---|
| Entry | Aryl halide | Product | Time (min)/Cyclesb | Conversionc (%) |
| 1 |
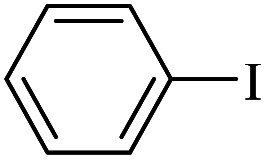
|
10a | 70/5 | 96 |
| 2 |
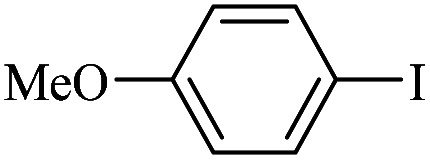
|
10b | 107/7 | 86 |
| 3 |

|
10c | 107/7 | 88 |
| 4 |
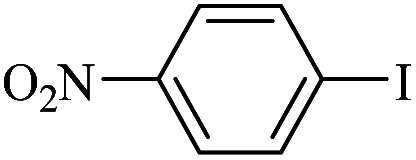
|
10d | 70/5 | 96 |
| 5 |
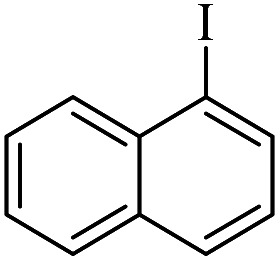
|
10e | 107/7 | 90 |
| 6 |
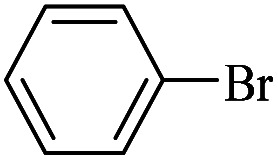
|
10a | 110/4 | 80 |
| 7 |

|
10b | 143/5 | 75 |
| 8 |
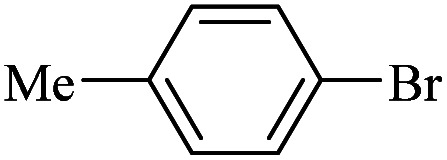
|
10c | 143/5 | 75 |
| 9 |
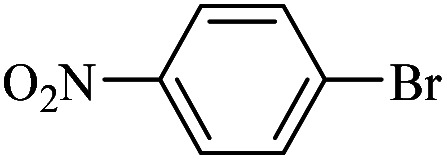
|
10d | 80/3 | 85 |
| 10 |
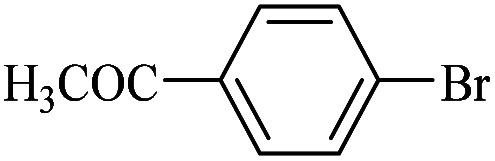
|
10f | 80/3 | 85 |
| 11 |
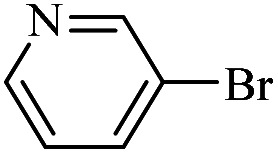
|
10g | 80/3 | 86 |
| 12 |
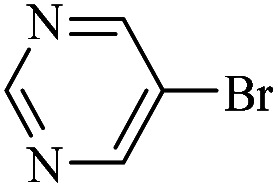
|
10h | 80/3 | 88 |
| 13 |
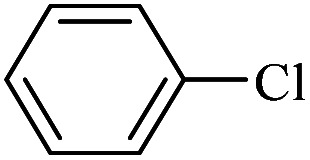
|
10a | 180/7 | 50 |
| 14 |

|
10b | 180/7 | N.R. |
| 15 |
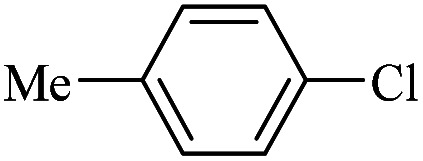
|
10c | 180/7 | 45 |
| 16 |

|
10f | 180/7 | 60 |
| 17 |

|
10d | 180/7 | 65 |
Reaction conditions: Phenylacetylene (1.0 mmol), iodobenzene (1.0 mmol), EtOH : H2O (2 : 1, v/v, 5.0 mL), 50 °C (on a water bath), N2 inlet (0.3 bar; 0.5 bar for aryl bromides, and chlorides), catalytic filter paper (7, placed on a glass funnel, containing 1.0 mmol Pd/95 cm2).
Total time spent in different cycles. Cycles refers to number of re-filtration of the residue (Scheme 2).
GC analysis.
