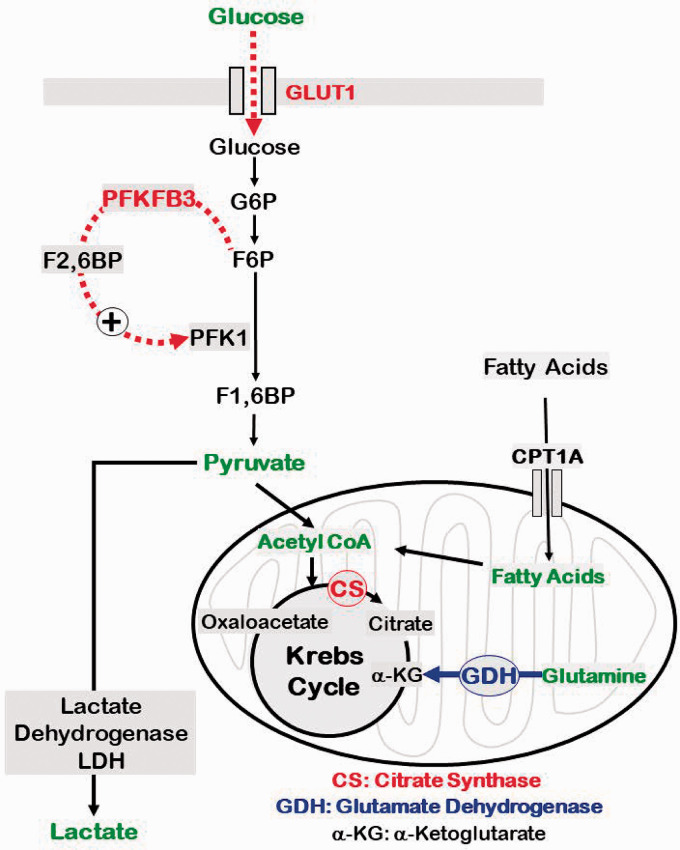
Figure 7.
Schematic of key impairments in the energy metabolism in aged BMVs. Impairments of glucose metabolism in aged BMVs involve decreased expression of GLUT1 and PFKFB3. GLUT1 is the primary transporter of glucose whereas PFKFB3 is a key activator of pyruvate generation in the glycolytic pathway. Interestingly, metabolism of pyruvate by lactate dehydrogenase (LDH) into lactate, the critical step in the anerobic respiration, was not altered in aged BMVs under the conditions of our study. On the other hand, metabolism of acetyl coenzyme A (CoA) into citrate by citrate synthase (CS) was reduced in aged BMVs. Formation of citrate from acetyl CoA is the first step in the Krebs cycle and reduced activity of CS thus could contribute to impaired OXPHOS in aged BMVs by reducing generation of electron donors (FADH2 and NADH). Notably, despite the above biochemical impairments in energy pathways, utilization of glucose or FAs were affected in aged BMVs. Interestingly, utilization of glutamine was found to be increased in aged BMVs. Consistent with this, we observed increased activity of GDH that catalyzes the conversion of α-KG from glutamine. α-KG is an intermediate in the Krebs cycle, increased flux of which could maintain the Krebs cycle activity in the presence of decreased CS activity. Thus, we identified several biochemical mechanisms contributing to the impaired energetics in aged BMVs that also include compensatory mechanisms. Enzymes/proteins highlighted in red indicate down regulation and highlighted in blue indicate upregulation in aged mice.