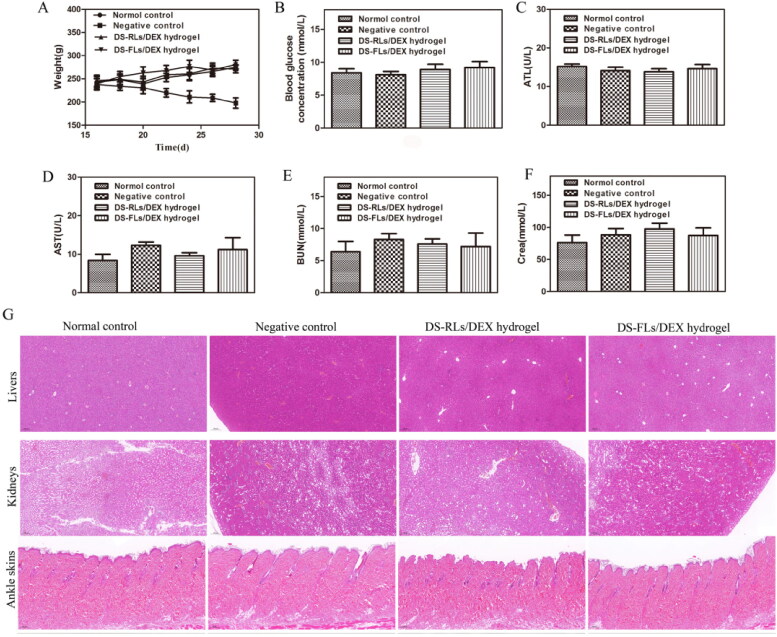
Figure 12.
Evaluation of biocompatibility of DS-RLs/DEX hydrogel and DS-FLs/DEX hydrogel in vivo. (A) Body weight. (B) Blood glucose concentration after 15 days of administration. (C) ALT, (D) AST, (E) BUN, and (F) Crea levels in serum. (G) HE staining histological sections of liver, kidney and ankle skin after DS-RLs/DEX hydrogel and DS-FLs/DEX hydrogel treatment (Scale bars = 200 nm).