Abstract
Introduction
Phosphodiesterase 4 (PDE4), which regulates inflammatory cytokine production leading to atopic dermatitis (AD), is selectively inhibited by difamilast. The objective of this phase III, long-term, open-label study was to evaluate the safety and efficacy of topical difamilast in Japanese adult and pediatric patients with AD.
Methods
Adult patients (n = 166) began treatment with difamilast 1% ointment, and pediatric patients began treatment with difamilast 0.3% ointment (n = 144) or difamilast 1% ointment (n = 56). Treatment was continued twice daily for 52 weeks. All patients had an Investigator’s Global Assessment (IGA) score of 2 (mild), 3 (moderate), or 4 (severe/very severe), and an AD-affected body surface area (BSA) of ≥ 5% before treatment, with no restriction on the upper limit for the AD-affected BSA.
Results
During therapy, 120 adult patients (72.3%) and 178 pediatric patients (89.0%) experienced treatment-emergent adverse events (TEAEs), most of which were mild or moderate in severity. Discontinuation due to TEAEs was reported in 13 adult patients (7.8%) and in 7 pediatric patients (3.5%). Treatment-related adverse events were reported in 14 adult patients (8.4%) and 16 pediatric patients (8.0%), most frequently dermatitis atopic (1.8%) and acne (1.2%) in adult patients and dermatitis atopic and pigmentation disorder (each 2.0%) in pediatric patients. The cumulative success rates in Eczema Area and Severity Index (EASI)-75 in adult and pediatric patients were 55.4% and 73.5%, respectively, at week 52, and the cumulative success rates increased from week 4 to week 52. The cumulative success rates in IGA score showed the same trend as those in EASI -75.
Conclusions
This study demonstrates that difamilast ointments are well tolerated and effective in Japanese adult and pediatric patients with AD when applied twice daily for 52 weeks, and are expected to be used for a long-term treatment for AD.
Clinical Trial Registration
Clinical Trials.gov identifier: NCT03961529.
Supplementary Information
The online version contains supplementary material available at 10.1007/s13555-022-00751-9.
Keywords: Application, Atopic dermatitis, Difamilast, Japanese adult and pediatric patients, Ointment, Open-label study, Phosphodiesterase 4, Safety
Key Summary Points
| Why carry out this study? |
| Difamilast selectively inhibits phosphodiesterase 4 (PDE4), an enzyme involved in the production of pro-inflammatory cytokines that lead to atopic dermatitis (AD). |
| This phase III, long-term, open-label study was conducted to confirm the safety and efficacy of topical difamilast in Japanese adult and pediatric patients with AD. |
| What was learned from the study? |
| This study demonstrates that difamilast ointments are well tolerated and effective in Japanese adult and pediatric patients with AD when applied twice daily for 52 weeks. |
| Most treatment-emergent adverse events (TEAEs) were mild or moderate in severity, and the discontinuation rates due to TEAEs were generally low in both adult and pediatric patients. |
| Difamilast ointments are expected to be a long-term treatment option for AD because of the safety and efficacy profiles. |
Introduction
Atopic dermatitis (AD) is a common chronic and relapsing inflammatory skin disease characterized by pruritus and xerosis. The prevalence rate ranges between 10% and 25% in children, and between 2% and 10% in adults in developed countries. AD can negatively affect a patient’s quality of life and psychosocial well-being [1–3].
Topical therapies are commonly used in treating AD, including moisturizers and emollients to recover skin barrier function, and anti-inflammatory agents to suppress inflammation [4, 5]. Topical corticosteroids (TCSs) and topical calcineurin inhibitors (TCIs), such as tacrolimus and pimecrolimus, are widely used but they can cause adverse events (AEs) that often prevent their long-term use in AD [2, 6]. Thus, there is a need for a new topical treatment for long-term use.
Phosphodiesterase 4 (PDE4) is an enzyme involved in the production of pro-inflammatory cytokines that lead to AD [2, 6]. Crisaborole is the first PDE4 inhibitor approved for AD treatment in patients aged ≥ 2 years by the US Food and Drug Administration (FDA, 2016), and then by the European Medicines Agency (2020). In 2020, the FDA approved a supplemental New Drug Application that expanded the use of crisaborole to include children ≥ 3 months of age [7].
Difamilast, a selective PDE4 inhibitor developed by Otsuka Pharmaceutical Co., Ltd. (Tokyo, Japan) [8], has shown favorable efficacy and safety profiles in the phase II studies conducted in Japan and the USA [9–12]. Furthermore, difamilast has shown superior efficacy to vehicle and was well tolerated in Japanese phase III studies, leading to its marketing approval recently in Japan [13, 14]. Here, we report a phase III, long-term, open-label study to evaluate the safety and efficacy of topical difamilast in Japanese adult and pediatric patients with AD.
Methods
This phase III, long-term, open-label study, conducted at 37 study sites in Japan between May 2019 and November 2020 (Clinical Trials.gov identifier: NCT03961529), was approved by the institutional review board at each study site. The study conformed to the principles expressed in the Declaration of Helsinki, the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use Good Clinical Practice Consolidated Guideline, and the applicable local laws and regulatory requirements in Japan. Patients aged ≥ 20 years provided written informed consent before participation in the study. For patients aged 15–20 years, written informed consent was obtained from both the patient and his/her legal guardian. For patients aged < 15 years, written informed consent was obtained from their legal guardian, and when possible, assent was obtained from the patient.
Patients
Male and female adult (aged 15–70 years) and pediatric (aged 2–14 years) outpatients diagnosed with AD according to the criteria of the Japanese Dermatological Association [15] with an Investigator’s Global Assessment (IGA) score (Table S1 in Supplementary Material) [16] of at least 2 (mild) and with an AD-affected body surface area [17] (BSA; excluding the scalp) of at least 5% at baseline were included in this study. The study enrolled patients with an IGA score of 4 (severe/very severe), with no restriction on the upper limit for the AD-affected BSA. Thus, this study included patients with more severe disease compared with those in the previous phase III studies [13, 14], closely reflecting the real-world clinical setting in Japan.
Patients with a history of a skin disease other than AD; clinically significant abnormal laboratory tests, blood pressure, or pulse rate; active viral skin infection; AD or contact dermatitis flare-up within 28 days prior to the baseline examination; or unable to stop other therapies for AD were excluded. Corticosteroids and immunosuppressants as eye, nasal, or ear drops, or as inhalants were permitted if their use was considered by the investigators to have no effect on AD assessments. Full exclusion criteria are described in Table S2 in Supplementary Material.
Study Design
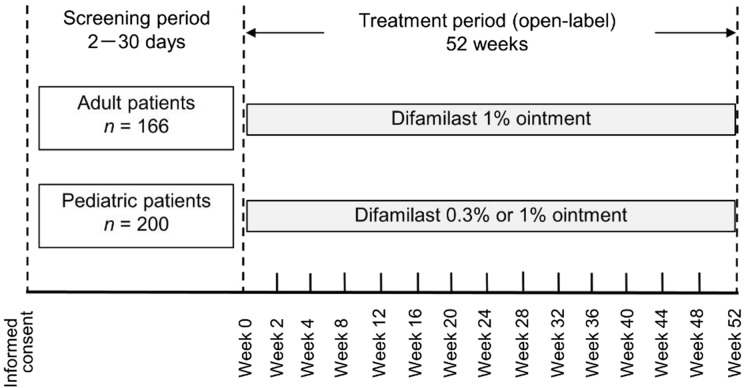
The study consisted of a screening period (2–30 days) and a treatment period (52 weeks). Safety and efficacy were examined on day 0 (baseline), at weeks 2 and 4, and every 4 weeks thereafter (Fig. 1). Patients were allowed to discontinue treatment at any time for any reason.
Fig. 1.
Study design
Adult and pediatric patients received difamilast ointments twice daily for 52 weeks. Adult patients received difamilast 1% ointment; the investigator determined which pediatric patient would receive either difamilast 0.3% or 1% ointment on the basis of their AD severity. The choice of difamilast ointments for pediatric patients could be changed on the basis of their efficacy or AEs during the treatment period. At each visit, the investigator instructed the patient (and for children, the legal guardian) to apply difamilast to the currently affected areas of skin excluding the scalp. The method used to calculate the dose for each patient has been reported previously [13, 14]. If needed, interruption or resumption of difamilast application was permitted for all patients on the basis of an existing condition.
Topical anti-inflammatory agents, TCSs and tacrolimus, could be used for the treatment of worsening of AD at the investigator’s discretion. However, concomitant use of these anti-inflammatory agents to the same application site as difamilast was prohibited.
Assessments
Assessment results were summarized for adult and pediatric patient groups. Patients visited the study sites fundamentally every 4 weeks, and safety was mainly evaluated. Safety assessments were based on symptoms, signs, clinical laboratory tests, and vital signs. A treatment-emergent adverse event (TEAE) was defined as any event that occurred during the treatment period irrespective of the relation with difamilast ointment. A treatment-related adverse event (TRAE) was defined as an event observed during the treatment period that was judged by the investigator to be related to difamilast ointment. The severity of an AE was classified as mild, moderate, or severe. AEs were coded to preferred terms using the Medical Dictionary for Regulatory Activities version 22.1. An AE coded as dermatitis atopic represented worsening of AD.
For difamilast application, the mean treatment period, total application amount, and applied amount per application were summarized in all patients. The number and percentage of patients who used topical anti-inflammatory agents for the treatment of worsening of AD were also recorded.
Efficacy assessments included the success rate in Eczema Area and Severity Index (EASI)-75 [18], defined as the percentage of patients achieving ≥ 75% improvement in overall EASI score from baseline, and that in IGA score, defined as the percentage of patients achieving an IGA score of 0 or 1 with at least 2-grade improvement. The mean percent change in overall EASI score from baseline was also evaluated.
The overall EASI score was calculated on the basis of the symptoms of the four body regions [face, neck, and head (excluding scalp); upper limbs; trunk; and lower limbs], ranging 0–72. The IGA score was assessed by a 5-point scale ranging from 0 (clear) to 4 (severe/very severe) (Table S1 in Supplementary Material) [16].
Statistical Analysis
Because this is a long-term, open-label study, statistical calculation of the sample size was not performed. No statistical tests were prespecified for comparison between the treatment groups. All patients who received difamilast at least once were included in both safety and efficacy analysis set. Safety and efficacy variables were analyzed descriptively using all observed data.
Results
Patients
Of the 381 patients screened, 366 patients were eligible and included in the study (adult patients, n = 166; pediatric patients, n = 200). Among the pediatric patients, 144 began with difamilast 0.3% ointment and 56 with difamilast 1% ointment. Overall, 302 patients completed the study [adult patients, n = 124 (74.7%); pediatric patients, n = 178 (89.0%)]. The most common reasons for discontinuation were withdrawal by patients (10.2%) and AEs (7.8%) in adult patients and withdrawal by legal guardian or AEs (each 3.5%) in pediatric patients (Table 1).
Table 1.
Patient disposition
| Adult patients (n = 166) | Pediatric patients (n = 200) | |
|---|---|---|
| Patients who completed the study | 124 (74.7) | 178 (89.0) |
| Patients who discontinued the study | 42 (25.3) | 22 (11.0) |
| Adverse event | 13 (7.8) | 7 (3.5) |
| Withdrawal by patient | 17 (10.2) | 3 (1.5) |
| Withdrawal by legal guardian | 0 (0.0) | 7 (3.5) |
| Physician decision | 2 (1.2) | 2 (1.0) |
| Lost to follow-up | 2 (1.2) | 1 (0.5) |
| Lack of efficacy | 2 (1.2) | 1 (0.5) |
| Other | 6 (3.6) | 1 (0.5) |
Data are number of patients (%) unless otherwise indicated
At study entry, 129 patients (35.2%) had an IGA score of 2, 210 (57.4%) had an IGA score of 3, and 27 (7.4%) had an IGA score of 4. On the basis of the Japanese AD severity index [19], 48 patients (13.1%) were rated as mild severity, 190 (51.9%) as moderate, 97 (26.5%) as severe, and 31 (8.5%) as very severe (Table 2).
Table 2.
Baseline patient characteristics, extent of exposure to difamilast ointment, and concomitant use of topical anti-inflammatory agents
| Adult patients (n = 166) | Pediatric patients (n = 200) | |
|---|---|---|
| Age, years, mean ± SD | 33.7 ± 11.3 | 7.4 ± 3.5 |
| Male | 101 (60.8) | 119 (59.5) |
| Years since onset of AD, mean ± SD | 24.7 ± 13.5 | 5.9 ± 3.5 |
| IGA score | ||
| IGA 2, mild disease | 51 (30.7) | 78 (39.0) |
| IGA 3, moderate disease | 102 (61.4) | 108 (54.0) |
| IGA 4, severe/very severe disease | 13 (7.8) | 14 (7.0) |
| Severity of ADa | ||
| Mild | 17 (10.2) | 31 (15.5) |
| Moderate | 91 (54.8) | 99 (49.5) |
| Severe | 45 (27.1) | 52 (26.0) |
| Very severe | 13 (7.8) | 18 (9.0) |
| EASI score, mean ± SD | 16.7 ± 10.6 | 12.6 ± 10.1 |
| Affected body surface area | ||
| ≥ 5% to < 10% | 12 (7.2) | 24 (12.0) |
| ≥ 10% to < 20% | 36 (21.7) | 57 (28.5) |
| ≥ 20% to < 40% | 62 (37.3) | 55 (27.5) |
| ≥ 40% | 56 (33.7) | 64 (32.0) |
| Exposure to difamilast ointment | ||
| Treatment period, days, mean ± SD | 274.5 ± 126.3 | 313.2 ± 96.7 |
| Total amount applied, g, mean ± SD | 1854.0 ± 1812.8 | 1332.8 ± 1141.0 |
| Amount applied per application, g, mean ± SD | 3.7 ± 2.9 | 2.3 ± 2.0 |
| Patients who used topical anti-inflammatory agents | 125 (75.3) | 150 (75.0) |
Data are number of patients (%) unless otherwise indicated
AD atopic dermatitis, EASI Eczema Area and Severity Index, IGA Investigator’s Global Assessment, SD standard deviation
aJapanese severity index of AD is based on the “Guidelines for the Treatment of Atopic Dermatitis 2008” by the Health and Labour Sciences Research
The mean treatment period with difamilast ointment was 274.5 days in adult patients and 313.2 days in pediatric patients. The mean amounts of difamilast ointment applied per application were 3.7 g in adult patients and 2.3 g in pediatric patients. The mean total amounts of difamilast ointment applied were 1854.0 g in adult patients and 1332.8 g in pediatric patients. Owing to worsening of AD, topical anti-inflammatory agents were used in 125 (75.3%) adult patients and 150 (75.0%) pediatric patients (Table 2).
Safety Assessment
Table 3 summarizes the overall TEAEs, and TEAEs observed in at least 5% of patients in any treatment group. Overall, 120 adult patients (72.3%) and 178 pediatric patients (89.0%) experienced TEAEs. Most TEAEs were mild or moderate in severity, except for one AE each of rhegmatogenous retinal detachment, diffuse large B-cell lymphoma, and pruritus in adult patients. The most frequent AEs were dermatitis atopic (21.1%), followed by nasopharyngitis (14.5%) in adult patients, and nasopharyngitis (32.0%), followed by dermatitis atopic (23.5%), and impetigo (15.5%) in pediatric patients.
Table 3.
Summary of overall treatment-emergent adverse events (TEAEs) and TEAEs observed in at least 5% of patients in any treatment group
| Adult patients (n = 166) | Pediatric patients (n = 200) | |
|---|---|---|
| Overall TEAEs | ||
| Patients with any TEAEs | 120 (72.3) | 178 (89.0) |
| Patients with mild TEAEs | 90 (54.2) | 134 (67.0) |
| Patients with moderate TEAEs | 27 (16.3) | 44 (22.0) |
| Patients with severe TEAEs | 3 (1.8) | 0 (0.0) |
| Patients with serious TEAEs | 2 (1.2) | 1 (0.5) |
| Deaths | 0 (0.0) | 0 (0.0) |
| TEAEs observed in at least 5% of patients in any treatment group | ||
| Eye disorders | ||
| Conjunctivitis allergic | 5 (3.0) | 21 (10.5) |
| Infections and infestations | ||
| Influenza | 5 (3.0) | 25 (12.5) |
| Gastroenteritis | 5 (3.0) | 17 (8.5) |
| Pharyngitis | 0 (0.0) | 10 (5.0) |
| Bronchitis | 1 (0.6) | 12 (6.0) |
| Nasopharyngitis | 24 (14.5) | 64 (32.0) |
| Molluscum contagiosum | 0 (0.0) | 10 (5.0) |
| Impetigo | 2 (1.2) | 31 (15.5) |
| Folliculitis | 10 (6.0) | 14 (7.0) |
| Neoplasms benign, malignant, and unspecified (including cysts and polyps) | ||
| Skin papilloma | 4 (2.4) | 10 (5.0) |
| Skin and subcutaneous tissue disorders | ||
| Acne | 8 (4.8) | 9 (4.5) |
| Dermatitis atopic | 35 (21.1) | 47 (23.5) |
| Dermatitis contact | 5 (3.0) | 12 (6.0) |
| Urticaria | 8 (4.8) | 17 (8.5) |
Data are number of patients (%) unless otherwise indicated. TEAEs were coded to preferred terms according to the Medical Dictionary for Regulatory Activities (MedDRA) version 22.1
TEAEs treatment-emergent adverse events
Serious AEs were observed in two adult patients (rhegmatogenous retinal detachment and diffuse large B-cell lymphoma) and in one pediatric patient (bacterial pneumonia); these events were assessed by the investigator as unrelated to difamilast. Rhegmatogenous retinal detachment was resolved by interruption of difamilast application, and bacterial pneumonia was resolved without changing the dose of difamilast. However, the AE of diffuse large B-cell lymphoma led to treatment discontinuation.
Discontinuation due to TEAEs was reported in 13 adult patients (7.8%) and in 7 pediatric patients (3.5%). The most common TEAEs leading to discontinuation were dermatitis atopic (1.8%), followed by pruritus or contact dermatitis (each 1.2%) in adult patients, and dermatitis atopic (2.5%) in pediatric patients (Table 4).
Table 4.
Treatment-emergent adverse events and treatment-related adverse events leading to discontinuation
| Adverse events leading to discontinuation | Adult patients (n = 166) | Pediatric patients (n = 200) | ||
|---|---|---|---|---|
| TEAEs | TRAEs | TEAEs | TRAEs | |
| 13 (7.8) | 5 (3.0) | 7 (3.5) | 4 (2.0) | |
| General disorders and administration site conditions | 1 (0.6) | 0 (0.0) | ||
| Malaise | 1 (0.6) | 0 (0.0) | ||
| Psychiatric disorders | 1 (0.6) | 0 (0.0) | ||
| Sleep disorder | 1 (0.6) | 0 (0.0) | ||
| Skin and subcutaneous tissue disorders | 10 (6.0) | 5 (3.0) | 7 (3.5) | 4 (2.0) |
| Pruritus | 2 (1.2) | 1 (0.6) | 0 (0.0) | 0 (0.0) |
| Dermatitis atopic | 3 (1.8) | 1 (0.6) | 5 (2.5) | 2 (1.0) |
| Pruritus allergic | 0 (0.0) | 0 (0.0) | 1 (0.5) | 1 (0.5) |
| Post-inflammatory pigmentation change | 1 (0.6) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Erythema | 1 (0.6) | 1 (0.6) | 0 (0.0) | 0 (0.0) |
| Dermatitis contact | 2 (1.2) | 1 (0.6) | 1 (0.5) | 1 (0.5) |
| Skin burning sensation | 1 (0.6) | 1 (0.6) | 0 (0.0) | 0 (0.0) |
| Neoplasms benign, malignant, and unspecified (including cysts and polyps) | 1 (0.6) | 0 (0.0) | ||
| Diffuse large B-cell lymphoma | 1 (0.6) | 0 (0.0) | ||
Data are number of patients (%) unless otherwise indicated. TEAEs and TRAEs were coded to preferred terms according to the Medical
Dictionary for Regulatory Activities (MedDRA) version 22.1
TEAEs treatment-emergent adverse events, TRAEs treatment-related adverse events
AEs were reported by the investigators as related to difamilast ointment in 14 adult patients (8.4%) and 16 pediatric patients (8.0%). The incidence of TRAEs was generally low in both patients. The most frequent TRAEs were dermatitis atopic (1.8%) and acne (1.2%) in adult patients, and dermatitis atopic and pigmentation disorder (each 2.0%) in pediatric patients. The incidence of infectious TRAEs was also low in both patient groups. Among them, treatment-related folliculitis was reported in two pediatric patients. The gastrointestinal TRAEs that have been observed with oral PDE4 inhibitors [20, 21] were not reported in this study of difamilast (Table 5). TRAEs leading to discontinuation are also summarized in Table 4. Dermatitis atopic as a TRAE leading to discontinuation was reported in two (1.0%) pediatric patients.
Table 5.
Treatment-related adverse events observed in each treatment group
| Adult patients (n = 166) | Pediatric patients (n = 200) | |
|---|---|---|
| Patients with any TRAEs | 14 (8.4) | 16 (8.0) |
| General disorders and administration site conditions | 1 (0.6) | 1 (0.5) |
| Application site pruritus | 1 (0.6) | 0 (0.0) |
| Application site pain | 0 (0.0) | 1 (0.5) |
| Infections and infestations | 1 (0.6) | 4 (2.0) |
| Kaposi varicelliform eruption | 0 (0.0) | 1 (0.5) |
| Tinea infection | 0 (0.0) | 1 (0.5) |
| Folliculitis | 1 (0.6) | 2 (1.0) |
| Skin and subcutaneous tissue disorders | 12 (7.2) | 11 (5.5) |
| Acne | 2 (1.2) | 0 (0.0) |
| Pruritus | 1 (0.6) | 1 (0.5) |
| Dermatitis atopic | 3 (1.8) | 4 (2.0) |
| Pruritus allergic | 0 (0.0) | 1 (0.5) |
| Post-inflammatory pigmentation change | 1 (0.6) | 0 (0.0) |
| Angioedema | 1 (0.6) | 0 (0.0) |
| Erythema | 1 (0.6) | 0 (0.0) |
| Pigmentation disorder | 1 (0.6) | 4 (2.0) |
| Dermatitis contact | 1 (0.6) | 1 (0.5) |
| Skin burning sensation | 1 (0.6) | 0 (0.0) |
| Skin hyperpigmentation | 0 (0.0) | 1 (0.5) |
Data are number of patients (%) unless otherwise indicated. TRAEs were coded to preferred terms according to the Medical Dictionary for Regulatory Activities (MedDRA) version 22.1
TRAEs treatment-related adverse events
No clinically problematic systemic or application site TRAEs were reported in this 52-week study. No deaths were reported, and no clinically relevant abnormalities were observed in clinical laboratory or vital sign assessments.
Efficacy Assessment
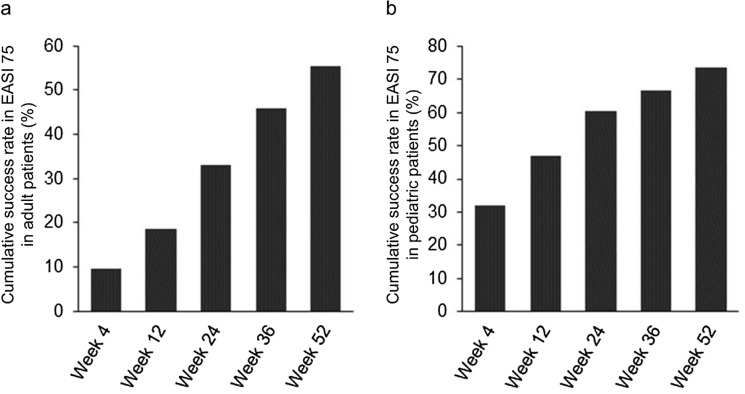
The cumulative success rates in EASI-75 at week 4 were 9.6% in adult patients and 32.0% in pediatric patients, with rates increasing to 33.1% and 60.5%, respectively, at week 24, and 55.4% and 73.5%, respectively, at week 52 (Fig. 2).
Fig. 2.
Cumulative success rate in Eczema Area and Severity Index (EASI)-75 a in adult patients (n = 166) and b in pediatric patients (n = 200). The data represent the cumulative percentage of patients achieving ≥ 75% improvement in overall EASI score from baseline
The cumulative success rates in IGA score in adult and pediatric patients, respectively, were 3.6% and 15.5% at week 4, 18.7% and 38.5% at week 24, and 34.9% and 52.5% at week 52 (Fig. S1 in Supplementary Material).
The mean percent changes in overall EASI score from baseline to week 4 were −21.3% in adult patients and −37.3% in pediatric patients; further improvements occurred by week 24 (−45.2% and −44.7%), and by week 52 (−59.6% and −61.2%; Fig. S2 in Supplementary Material).
Discussion
The long-term treatment with difamilast, a new topical PDE4 inhibitor, in this study was well tolerated in Japanese adult and pediatric patients aged ≥ 2 years with AD. No new safety concerns were identified during difamilast application for 52 weeks. Namely, safety profiles of difamilast in this study were consistent with those in the previous clinical studies conducted in Japan [10, 12–14]. The overall discontinuation rates were 25.3% in adult patients and 11.0% in pediatric patients, and most patients could complete the 52-week treatment with difamilast ointments. Although 120 adult patients (72.3%) and 178 pediatric patients (89.0%) experienced TEAEs, most TEAEs were mild or moderate in severity. Therefore, the discontinuation rates due to TEAEs were low in adult (7.8%) and pediatric (3.5%) patients, and difamilast ointments are considered to be usable for a long-term treatment of AD. Furthermore, the incidence of TRAEs was generally low in both adult and pediatric patients (8.4% and 8.0%, respectively) in spite of the somewhat high incidence of TEAEs.
Unlike the effects observed with other PDE4 inhibitors [22], no skin stinging or burning sensations were observed in pediatric patients using either concentration of difamilast in this study; treatment-related mild skin burning sensation was reported by one adult patient. Skin burning sensations were frequently reported in a 6-month study of tacrolimus 0.1% ointment, although the incidence decreased after the first week of treatment [23]. Nasopharyngitis was frequently reported but was unrelated to treatment in this study of topical difamilast.
Atopic skin is associated with secondary bacterial, viral, and fungal skin infections due to epidermal barrier dysfunction and immune dysregulation [1, 5]. The incidence of treatment-related infectious AEs was generally low in both adult and pediatric patients (0.6% and 2.0%, respectively). Impetigo, unrelated to treatment, was observed mainly among the pediatric patients, which is reported to be associated with dry skin with AD in the pediatric population [24, 25]. Treatment-related folliculitis was observed in only one adult patient (0.6%) and two pediatric patients (1.0%) in this study, and all cases were rated as mild in severity. In contrast, folliculitis is often found when using TCSs classified as strong or higher according to the Japanese Dermatological Association’s criteria [26, 27], or when using topical tacrolimus (reported incidence of 11.7%) [23]. Kaposi varicelliform eruption (KVE), a skin infection caused by herpes simplex virus in patients with AD [28], was observed in one adult patient (0.6%) and in three pediatric patients (1.5%); all cases were rated as mild or moderate in severity. KVE was thought to be related to treatment in only one pediatric patient (0.5%).
The PDE4 inhibitor apremilast [29] is approved for oral treatment of psoriasis and psoriatic arthritis, as is roflumilast [30] for the management of exacerbation in severe chronic obstructive pulmonary disease. These agents have been reported to be associated with gastrointestinal AEs [20, 21]. No treatment-related gastrointestinal AEs were observed in this study of topical difamilast application.
The efficacy results herein are consistent with those of the previous studies [9–14]. Our data show cumulative success rates in EASI-75 increasing from week 4 to week 52 in both adult and pediatric patients, and cumulative success rates in IGA score showing the same trend. For the observed case (OC) analysis as further data, the success rates in EASI-75 at week 52 in this study were 47.2% (58/123) in adult patients and 46.3% (82/177) in pediatric patients. Thus, the patients who achieved ≥ 75% improvement in overall EASI score at week 52 were substantially observed in the OC analysis as well. Furthermore, a substantial decrease in the mean EASI score from baseline was observed at week 4 in adult and pediatric patients, with further continued improvement through week 52. The safety and efficacy profiles of topical difamilast may be due to the stronger inhibitory activity against PDE4B than the other three PDE4 subtypes and the degree of its cutaneous absorption [31, 32].
The present study has some limitations. First, the lack of the control group limits the interpretation of the study results of difamilast ointments. Second, the use of topical anti-inflammatory agents allowing for worsening of AD also limits discussions on the long-term study results of difamilast. Finally, only Japanese patients were included in the study, and patients aged < 2 years with a higher prevalence of AD were not included. Therefore, it is not certain whether the results are applicable to non-Japanese patients. In contrast, a phase 3 study of difamilast ointment is currently being planned in Japanese patients with AD aged < 2 years.
Conclusions
Although this study has some limitations, difamilast ointments are well tolerated and effective in Japanese adult and pediatric patients with AD when used twice daily for 52 weeks, and are expected to be used for long-term treatment of AD. Difamilast is the first PDE4 inhibitor to receive manufacturing and marketing approval in Japan on 27 September 2021 for the indication of AD in adult and pediatric (aged ≥ 2 years) patients. Given that approximately 60% of patients are known to develop AD during their first year of life [4, 6], future studies should focus on patients with AD aged < 2 years.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors sincerely thank all study investigators (Table S3 in Supplementary Material) and personnel for their effort and contribution to this study, and all patients who participated in the study. The authors also thank all members of the project team at Otsuka Pharmaceutical Co., Ltd. who were involved in the study. Furthermore, the authors sincerely thank Dr. Jon M. Hanifin, Dr. Charles N. Ellis, and Dr. Lawrence F. Eichenfield for their review of the manuscript.
Funding
This study was supported by Otsuka Pharmaceutical Co., Ltd. (Tokyo, Japan). The journal’s Rapid Service Fee was also sponsored by Otsuka Pharmaceutical Co., Ltd. (Tokyo, Japan).
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
Hidehisa Saeki, Tomomi Imamura, and Daisuke Yokota contributed to the concept and design of the study, and data acquisition. Daisuke Yokota and Hidetsugu Tsubouchi participated in data analysis, and all authors were involved in the interpretation of the data. Hidetsugu Tsubouchi wrote the first draft of the manuscript, and all authors reviewed the previous versions of the manuscript and approved the final manuscript before submission.
Medical Writing and Editorial Assistance
Medical writing and editorial support in the preparation of this article was provided by Hidetsugu Tsubouchi, and was funded by Otsuka Pharmaceutical Co., Ltd. (Tokyo, Japan) according to the Good Publication Practice guideline.
Disclosures
Hidehisa Saeki was the medical expert for this study, and has received fees for consultation from Otsuka Pharmaceutical Co., Ltd. (Tokyo, Japan). Furthermore, Hidehisa Saeki has received funding or grant support from Eisai, Maruho, Mitsubishi Tanabe Pharma, and Torii Pharmaceutical, and honorarium as a consultant from AbbVie, LEO Pharma, and Sanofi. Tomomi Imamura, Daisuke Yokota, and Hidetsugu Tsubouchi are employees of Otsuka Pharmaceutical Co., Ltd.
Compliance with Ethics Guidelines
This study was approved by the respective institutional review boards at each study site (investigators and study sites are listed in Supplementary Table S3), and conducted in accordance with the ethical principles outlined in the Declaration of Helsinki, the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use Good Clinical Practice Consolidated Guideline, and the applicable local laws and regulatory requirements in Japan. All patients or their legal guardians provided written informed consent before participation in the study.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
- 1.Pavlis J, Yosipovitch G. Management of itch in atopic dermatitis. Am J Clin Dermatol. 2018;19:319–332. doi: 10.1007/s40257-017-0335-4. [DOI] [PubMed] [Google Scholar]
- 2.Nygaard U, Deleuran M, Vestergaard C. Emerging treatment options in atopic dermatitis: topical therapies. Dermatology. 2017;233:333–343. doi: 10.1159/000484407. [DOI] [PubMed] [Google Scholar]
- 3.Mollanazar NK, Smith PK, Yosipovitch G. Mediators of chronic pruritus in atopic dermatitis: getting the itch out? Clin Rev Allergy Immunol. 2016;51:263–292. doi: 10.1007/s12016-015-8488-5. [DOI] [PubMed] [Google Scholar]
- 4.Bieber T. Atopic dermatitis. Ann Dermatol. 2010;22:125–137. doi: 10.5021/ad.2010.22.2.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bieber T. Atopic dermatitis. N Engl J Med. 2008;358:1483–1494. doi: 10.1056/NEJMra074081. [DOI] [PubMed] [Google Scholar]
- 6.Silverberg JI, Nelson DB, Yosipovitch G. Addressing treatment challenges in atopic dermatitis with novel topical therapies. J Dermatol Treat. 2016;27:568–576. doi: 10.1080/09546634.2016.1174765. [DOI] [PubMed] [Google Scholar]
- 7.Fahrbach K, Tarpey J, Washington EB, et al. Crisaborole ointment, 2%, for treatment of patients with mild-to-moderate atopic dermatitis: systematic literature review and network meta-analysis. Dermatol Ther (Heidelb) 2020;10:681–694. doi: 10.1007/s13555-020-00389-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hiyama H, Arichika N, Sakurai K. Pharmacological activity of difamilast, a novel PDE4 inhibitor for the topical treatment of atopic dermatitis: comparison with other PDE4 inhibitors. Presented at: American Academy of Dermatology Annual Meeting; March 1–5, 2019; Washington, DC.
- 9.Hanifin JM, Ellis CN, Frieden IJ, et al. OPA-15406, a novel, topical, nonsteroidal, selective phosphodiesterase-4 (PDE4) inhibitor, in the treatment of adult and adolescent patients with mild to moderate atopic dermatitis (AD): a phase-II randomized, double-blind, placebo-controlled study. J Am Acad Dermatol. 2016;75:297–305. doi: 10.1016/j.jaad.2016.04.001. [DOI] [PubMed] [Google Scholar]
- 10.Saeki H, Kawashima M, Sugaya S, Oshiden K, Tsubouchi H. Efficacy and safety of topical OPA-15406, a new phosphodiesterase 4 inhibitor, in Japanese patients with atopic dermatitis for 8 weeks: a phase 2, randomized, double-blind, placebo-controlled study. J Dermatol. 2019;46:672–679. doi: 10.1111/1346-8138.14979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eichenfield LF, Rosenberg N, Roth S, Davis LA, Pariser DM. Efficacy and safety of difamilast, a topical PDE4 inhibitor, in a phase 2 study of pediatric patients with atopic dermatitis. Presented at: American Academy of Dermatology Annual Meeting; March 1–5, 2019; Washington, DC.
- 12.Saeki H, Baba N, Oshiden K, Abe Y, Tsubouchi H. Phase 2, randomized, double-blind, placebo-controlled, 4-week study to evaluate the safety and efficacy of OPA-15406 (difamilast), a new topical selective phosphodiesterase type-4 inhibitor, in Japanese pediatric patients aged 2–14 years with atopic dermatitis. J Dermatol. 2020;47:17–24. doi: 10.1111/1346-8138.15137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Saeki H, Baba N, Ito K, Yokota D, Tsubouchi H. Difamilast, a selective phosphodiesterase 4 inhibitor, ointment in paediatric patients with atopic dermatitis: a phase III randomised double-blind, vehicle-controlled trial. Br J Dermatol. 2022;186:40–49. doi: 10.1111/bjd.20655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Saeki H, Ito K, Yokota D, Tsubouchi H. Difamilast ointment in adult patients with atopic dermatitis: a phase 3 randomized double-blind vehicle-controlled trial. J Am Acad Dermatol. 2021;86:607–614. doi: 10.1016/j.jaad.2021.10.027. [DOI] [PubMed] [Google Scholar]
- 15.Saeki H. Management of atopic dermatitis in Japan. J Nippon Med Sch. 2017;84:2–11. doi: 10.1272/jnms.84.2. [DOI] [PubMed] [Google Scholar]
- 16.Futamura M, Leshem YA, Thomas KS, Nankervis H, Williams HC, Simpson EL. A systematic review of Investigator Global Assessment (IGA) in atopic dermatitis (AD) trials: many options, no standards. J Am Acad Dermatol. 2016;74:288–294. doi: 10.1016/j.jaad.2015.09.062. [DOI] [PubMed] [Google Scholar]
- 17.Mosteller RD. Simplified calculation of body-surface area. N Engl J Med. 1987;317:1098. doi: 10.1056/NEJM198710223171717. [DOI] [PubMed] [Google Scholar]
- 18.Hanifin JM, Thurston M, Omoto M, Cherill R, Tofte SJ, Graeber M. The eczema area and severity index (EASI): assessment of reliability in atopic dermatitis. EASI Evaluator Group. Exp Dermatol. 2001;10:11–18. doi: 10.1034/j.1600-0625.2001.100102.x. [DOI] [PubMed] [Google Scholar]
- 19.Katayama I, Aihara M, Ohya Y, et al. Japanese guidelines for atopic dermatitis 2017. Allergol Int. 2017;66:230–247. doi: 10.1016/j.alit.2016.12.003. [DOI] [PubMed] [Google Scholar]
- 20.Paul C, Cather J, Gooderham M, et al. Efficacy and safety of apremilast, an oral phosphodiesterase 4 inhibitor, in patients with moderate-to-severe plaque psoriasis over 52 weeks: a phase III, randomized controlled trial (ESTEEM 2) Br J Dermatol. 2015;173:1387–1399. doi: 10.1111/bjd.14164. [DOI] [PubMed] [Google Scholar]
- 21.Calverley PM, Rabe KF, Goehring UM, Kristiansen S, Fabbri LM, Martinez FJ, M2–124 and M2–125 study groups Roflumilast in symptomatic chronic obstructive pulmonary disease: two randomised clinical trials. Lancet. 2009;374:685–694. doi: 10.1016/S0140-6736(09)61255-1. [DOI] [PubMed] [Google Scholar]
- 22.Eichenfield LF, Call RS, Forsha DW, et al. Long-term safety of crisaborole ointment 2% in children and adults with mild to moderate atopic dermatitis. J Am Acad Dermatol. 2017;77:641–9.e5. doi: 10.1016/j.jaad.2017.06.010. [DOI] [PubMed] [Google Scholar]
- 23.Reitamo S, Ortonne JP, Sand C, et al. A randomized, double-blind, controlled study of long-term treatment with 0.1% tacrolimus ointment in adults with moderate to severe atopic dermatitis. Br J Dermatol. 2005;152:1282–1289. doi: 10.1111/j.1365-2133.2005.06592.x. [DOI] [PubMed] [Google Scholar]
- 24.Fenner J, Silverberg NB. Skin diseases associated with atopic dermatitis. Clin Dermatol. 2018;36:631–640. doi: 10.1016/j.clindermatol.2018.05.004. [DOI] [PubMed] [Google Scholar]
- 25.Furue M, Yamazaki S, Jimbow K, et al. Prevalence of dermatological disorders in Japan: a nationwide, cross-sectional, seasonal, multicenter, hospital-based study. J Dermatol. 2011;38:310–320. doi: 10.1111/j.1346-8138.2011.01209.x. [DOI] [PubMed] [Google Scholar]
- 26.Drake LA, Dinehart SM, Farmer ER, et al. Guidelines of care for the use of topical glucocorticosteroids. J Am Acad Dermatol. 1996;35:615–619. doi: 10.1016/S0190-9622(96)90690-8. [DOI] [PubMed] [Google Scholar]
- 27.Japanese Dermatological Association. Clinical Practice Guidelines for the Management of Atopic Dermatitis 2021. https://www.dermatol.or.jp/uploads/uploads/files/guideline/ADGL2021.pdf. [In Japanese].
- 28.Damour A, Garcia M, Seneschal J, Lévêque N, Bodet C. Eczema herpeticum: clinical and pathophysiological aspects. Clin Rev Allergy Immunol. 2020;59:1–18. doi: 10.1007/s12016-019-08768-3. [DOI] [PubMed] [Google Scholar]
- 29.Deeks ED. Apremilast: a review in psoriasis and psoriatic arthritis. Drugs. 2015;75:1393–1403. doi: 10.1007/s40265-015-0439-1. [DOI] [PubMed] [Google Scholar]
- 30.Yuan L, Dai X, Yang M, Cai Q, Shao N. Potential treatment benefits and safety of roflumilast in COPD: a systematic review and meta-analysis. Int J Chron Obstruct Pulmon Dis. 2016;11:1477–1483. doi: 10.2147/COPD.S106370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ahluwalia J, Udkoff J, Waldman A, Borok J, Eichenfield LF. Phosphodiesterase 4 inhibitor therapies for atopic dermatitis: progress and outlook. Drugs. 2017;77:1389–1397. doi: 10.1007/s40265-017-0784-3. [DOI] [PubMed] [Google Scholar]
- 32.Guttman-Yassky E, Hanifin JM, Boguniewicz M, et al. The role of phosphodiesterase 4 in the pathophysiology of atopic dermatitis and the perspective for its inhibition. Exp Dermatol. 2019;28:3–10. doi: 10.1111/exd.13817. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.