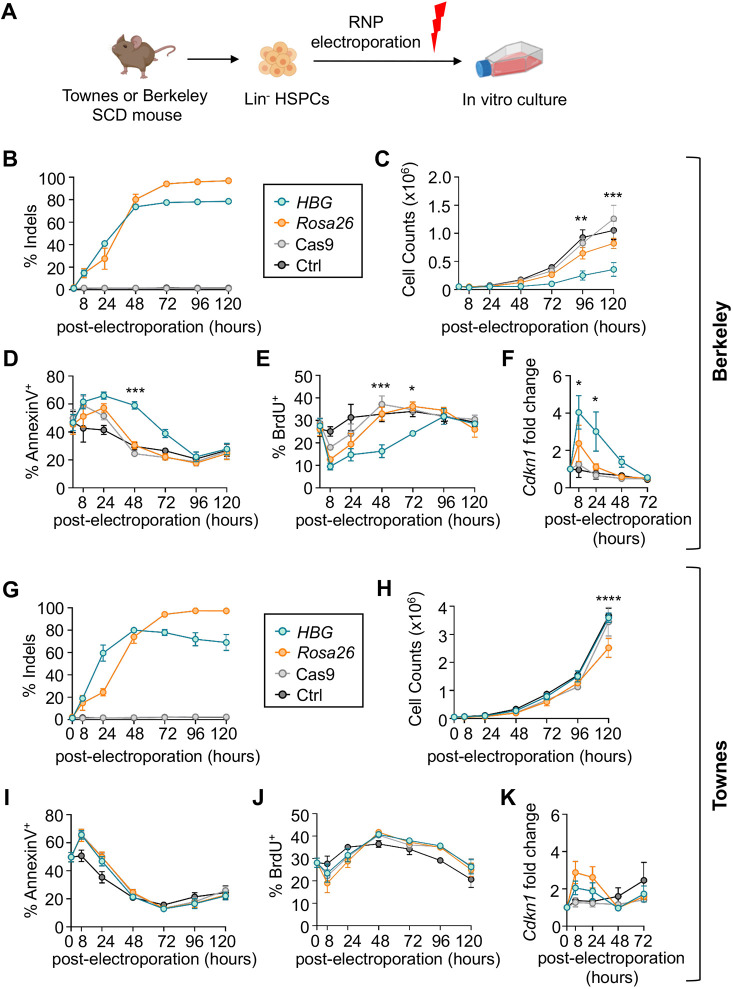
Fig. 3.
Editing the human γ-globin transgenes impairs the viability of Berkeley but not Townes mouse hematopoietic stem and progenitor cells (HSPCs). (A) Experimental strategy. Three million lineage-negative (Lin−) bone marrow cells from Berkeley (B-F) or Townes (G-K) mice were electroporated with ribonucleoprotein (RNP) complex consisting of Cas9+guide RNA (gRNA) targeting the −118 to −114 BCL11A binding site (TGACCA) in the HBG1/2 promoter, or a control gRNA targeting Rosa26 intron 1, and grown in culture for 5 days with mouse stem cell factor (mSCF), human FLT3 (hFLT3) ligand, mouse interleukin 3 (mIL-3) and mouse interleukin 11 (mIL-11). (B,G) On-target insertion-deletion (indel) mutations in genomic DNA, determined by next-generation sequencing (NGS) of PCR products generated across the targeted region after editing. Controls include electroporation with Cas9 alone or non-electroporated cells (Ctrl). (C,H) Live cell numbers measured by Trypan Blue exclusion. (D,I) Percentages of apoptotic (Annexin V+) cells measured by flow cytometry. (E,J) Percentages of S-phase cells measured by incorporation of bromodeoxyuridine (BrdU). (F,K) Fold change in the TP53-induced Cdkn1 (p21) mRNA measured by real-time quantitative PCR (RT-qPCR), normalized to mouse Gapdh mRNA. All graphs show data as mean±s.e.m. from two biological replicate experiments with cells from two mice for each replicate (n=4 mice total). *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001, by two-way ANOVA for HBG- versus Rosa26-edited cells.