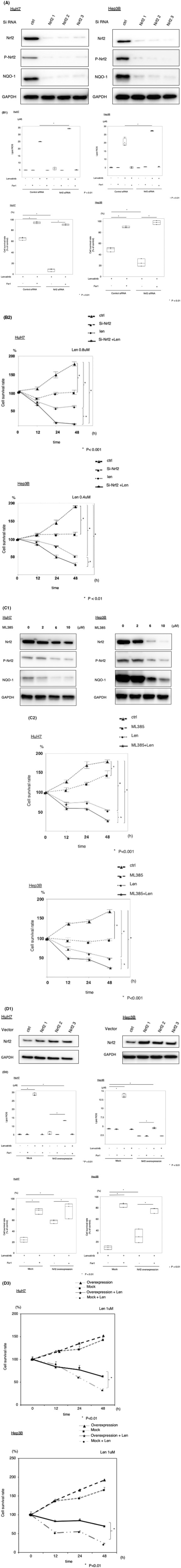
FIGURE 2.
Suppression of lenvatinib‐induced ferroptosis by nuclear factor erythroid‐derived 2‐like 2 (Nrf2) in hepatocellular carcinoma (HCC) cells. (A) HCC cells were transfected with siRNA (Nrf2 and control), and Nrf2, phosphorylated Nrf2 (p‐Nrf2), and NADPH quinone oxidoreductase 1 (NQO1) protein levels were assayed via immunoblotting. (B) HCC cells were transfected with siRNA (Nrf2 and control) and treated with lenvatinib (HuH7: 0.8 µM, Hep3B: 0.4 µM). Malondialdehyde (MDA) levels and cell viability were analyzed. The data are representative of at least three independent experiments. *P < 0.01 versus the control group. (C) HCC cells were treated with ML385, and Nrf2, p‐Nrf2, and NQO1 protein levels were assayed via immunoblotting. HCC cells were treated with ML385 and lenvatinib (HuH7: 0.8 µM, Hep3B: 0.4 µM). Cell viability was analyzed using the CellTiter‐Glo assay. Hep3B and HuH7 cells were transfected with empty vector or Nrf2 plasmids, and Nrf2 protein levels were assayed via immunoblotting. The transfected cells were treated with lenvatinib (HuH7: 0.8 µM, Hep3B: 0.4 µM) or ferrostatin‐1 (fer1, 10 µM). MDA levels were analyzed. Cell viability was analyzed using the CellTiter‐Glo assay. (D) HCC cells transfected with Nrf2 overexpression or control plasmids were treated with lenvatinib (HuH7: 0.8 µM, Hep3B: 0.4 µM). Viability was analyzed using the CellTiter‐Glo assay. Len, lenvatinib