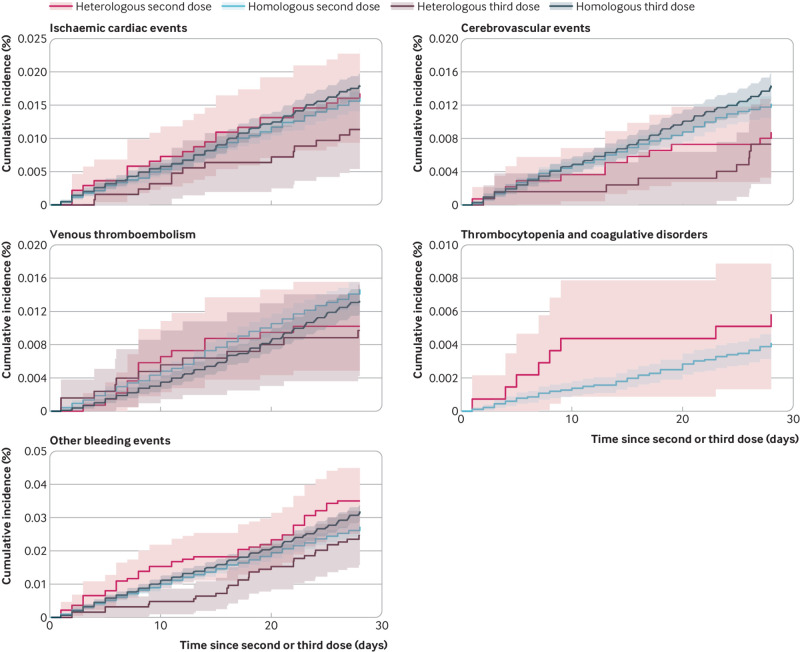
Fig 1.
Cumulative incidence curves of the cardiovascular and haemostatic adverse events for the heterologous and homologous primary and booster vaccine schedules. Cumulative incidence curves of the main outcomes within the first 28 days after the last day of vaccination for the heterologous vaccine schedules of ChAdOx1-S priming and mRNA booster dose or doses (ie, two or three doses) and homologous primary and booster mRNA vaccine schedules (ie, two or three doses) with the corresponding 95% confidence intervals (shaded areas). The number of events of arterial thromboembolisms and myocarditis or pericarditis, as well as for thrombocytopenia and coagulative disorder for the three dose comparison, were low to none, which is why cumulative incidence curves for these outcomes were not estimated. Other bleeding events include a composite of bleeding-related diagnoses other than intracranial haemorrhages