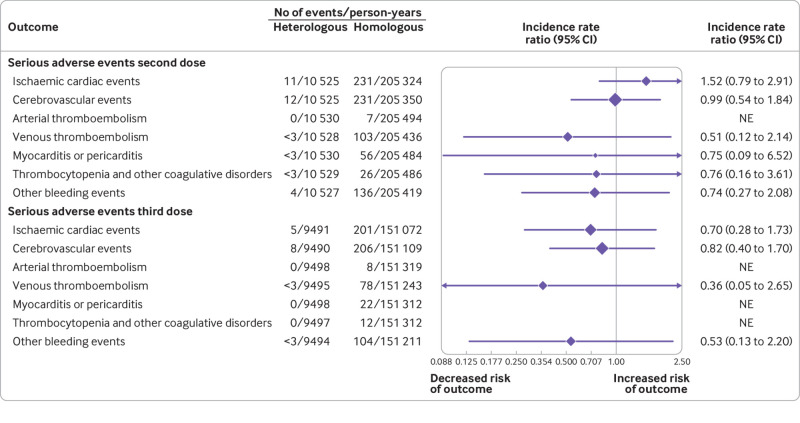
Fig 4.
Sensitivity analysis of the associated risk of serious adverse events with heterologous primary and booster vaccine schedules. Shows the results of the sensitivity analysis where restricting the outcome definitions to serious adverse events, defined as excluding brief hospital contacts of less than 24 h. Top panel shows the results of the primary (two dose) vaccine schedules comparison while the booster (three dose) schedules comparison is presented in the bottom panel. Incidence rate ratios (IRRs) were adjusted for calendar period, sex, birth year (proxy for age), region of residency, birth country, vaccine priority group, hospital contact in the last six months, and comorbidities. Other bleeding events includes a composite of bleeding-related diagnoses other than intracranial haemorrhages. CI=confidence interval; NE=not estimated