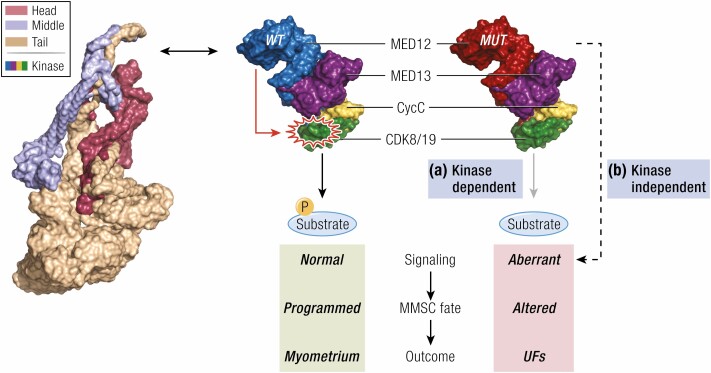
Figure 3.
Role of MED12 mutation in the pathogenesis of fibroids. Two mutually compatible models are demonstrating that fibroids driver mutations in MED12 trigger myometrial stem cell transformation and fibroids formation through altered signaling. In the first model (A), MED12 mutations in exon 2 disrupt the CDK8 T-loop conformation to affect Mediator kinase activity and the phosphorylation of downstream targets, including those that control myometrial stem cell fate and/or function. In the second model (B), MED12 mutations alter gene expression programs that control myometrial stem cell fate and/or function through kinase-independent mechanisms, such as MED12 interactions with transcriptional regulatory proteins (173). The 2 models are not mutually exclusive, and both scenarios could contribute to fibroids pathogenesis. Shown here is the 4-subunit Mediator kinase module comprising MED13, MED12, CycC, and CDK8/19 that variably associates with a core Mediator, which is collectively composed of 26 different subunits arranged into 3 structurally defined domains, ie, Head, Middle, and Tail. The structure of the core Mediator is from Clark et al (137), whereas that of the kinase module is from Li et al (167). Abbreviations: CDK8/19, cyclin-dependent kinase 8/19; CycC, cyclin C; MED12/13, RNA polymerase II transcriptional mediator complex subunit 12/13; MMSC, myometrial stem cell; UFs, uterine fibroids.