Abstract
Background
Solid organ transplant (SOT) recipients are at high risk for complications from coronavirus disease 2019 (COVID-19) and vaccine breakthrough infections are common. We determined the effectiveness of ≥3 doses of mRNA vaccine and early monoclonal antibody therapy in reducing disease severity against the Omicron (B.1.1.529) variant.
Methods
Prospective cohort study of consecutive SOT recipients with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection referred to our transplant center who were followed for at least 30 days. The primary outcome was supplemental oxygen requirement. Effectiveness of sotrovimab and ≥3 vaccine doses was estimated using adjusted risk ratios (RR).
Results
Three hundred adult organ transplant recipients were included. Seventy-one patients (24.1%) were hospitalized, 44 (14.9%) required supplemental oxygen, 19 (6.5%) were admitted to the intensive care unit (ICU), 15 (5.1%) required mechanical ventilation (MV), and 13 (4.4%) died. On multivariate analysis, age and multiple comorbidities were risk factors for oxygen requirement. Both receipt of ≥3 vaccine doses prior to SARS-CoV-2 infection and receipt of sotrovimab in the first 7 days of symptom onset was associated with a reduction in the need for supplemental oxygen (RR 0.30 [95% confidence interval {CI}: .17 to .54] and RR 0.24 (95% CI: .1 to .59), respectively]. For sotrovimab, the number needed to treat (NNT) to prevent one patient requiring oxygen was 6.64 (95% CI: 4.56–13.66). Both sotrovimab use and having received ≥3 vaccine doses were also associated with a shorter hospitalization length of stay.
Conclusions
In a cohort of SOT recipients with Omicron variant COVID-19 infection, prior receipt of ≥3 mRNA vaccine doses and early monoclonal antibody therapy were independently associated with significantly reduced disease severity.
Keywords: disease severity, immunosuppression, SARS-CoV-2, sotrovimab, mRNA vaccine
In an organ transplant population, this prospective study shows that being vaccinated with ≥3 doses of mRNA vaccine and using monoclonal antibody within 7 days of symptom onset are protective of severe outcomes in patients with vaccine breakthrough coronavirus disease 2019 (COVID-19).
Solid organ transplant (SOT) recipients are at high risk for complications from coronavirus disease 2019 (COVID-19) [1–3]. Several studies performed early in the pandemic suggest high rates of hospitalization of 50–70%, intensive care unit (ICU) admission rates of 20–30%, and mortality rates ranging 10–30% depending on type of organ transplanted. Lung transplant recipients appear to have the greatest severity. Transplant recipients were prioritized for vaccination and an analysis by the Centers for Disease Control and Prevention (CDC) showed that from January to September 2021, 2 doses of vaccine had an effectiveness of 59% in organ transplant recipients [4]. However, breakthrough infections continued to occur at a high rate [5]. A third dose of mRNA vaccine was subsequently recommended in August 2021 as part of a primary series, based on immunogenicity studies, and a fourth dose booster is now recommended [6, 7].
The treatment of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in transplant recipients has followed general population guidelines. Experience with early outpatient treatment for mild COVID-19 in transplant recipients includes monoclonal antibody therapy. Case series of transplant recipients treated primarily with casirivimab/imdevimab show that monoclonals are well-tolerated, reduce SARS-CoV-2 viral loads in the nasopharynx and that treated patients tend to have low rates of hospitalization [8, 9].
Although SARS-CoV-2 has evolved with multiple variants, the more recent Omicron variant (B.1.1.529) possesses mutations associated with increased transmission and diminished protection conferred by pre-existing immunity from vaccination or infection, as well as a decrease in neutralizing activity of some monoclonal antibodies (mAbs) [10]. Sotrovimab is a broadly neutralizing sarbecovirus mAb that retains potency in neutralizing Omicron [11]. Preliminary studies in the general population show a decreased severity of COVID-19 disease in the Omicron-driven wave [12]. However, data regarding the impact of Omicron variant on the severity of disease in the organ transplant population are limited. Moreover, with widespread Omicron infection and vaccine breakthrough infections occurring, there are limited data about the severity of this variant and the real-world effectiveness of vaccines and early monoclonal antibody therapy. Vaccination and early antiviral therapy for influenza infection has shown to attenuate disease severity in transplant recipients [13]. Therefore, in parallel, we hypothesized that SARS-CoV-2 vaccine and early sotrovimab may also impact the course of disease in transplant patients with COVID-19.
METHODS
Study Design
We conducted a single center prospective cohort study at the University Health Network Organ Transplant Program in Toronto, Canada. The program actively follows more than 7000 transplant patients and performs approximately 600 new transplants annually. Since March 2020, all organ transplant recipients registered with our center and who have a COVID-19 diagnosis have been prospectively enrolled in the COVID-19 registry. We continued to enroll patients during the Omicron variant wave. The current study reports on the first 300 patients at our center during the Omicron wave. In Ontario, >90% of SARS-CoV-2 infections were sequenced as Omicron variant by 15 December 2021 [14]. Therefore, we restricted this analysis to patients that were diagnosed starting on 15 December 2021. The last patient included was diagnosed on 24 January 2022 in order to have a minimum of 30-day follow-up on all patients.
Study Population
Eligible patients were adult organ transplant recipients with a confirmed diagnosis of COVID-19 during the Omicron wave period in Canada. Both rapid antigen test and polymerase chain reaction were accepted as confirmation tests. The study was approved by the University Health Network Research Ethics Board and a consent waiver was obtained for data collection. Baseline characteristics included: age at diagnosis, sex, and transplant information (type of transplant, time from transplantation, immunosuppressive therapy, the presence of rejection in the last 3 months). Comorbidities included: hypertension, diabetes, obesity (defined as body mass index [BMI]≥30), coronary artery disease, congestive cardiac failure, chronic lung disease, chronic kidney disease (defined as glomerular filtration rate <60 mL/min/1.73 m2), active systemic malignancy (including tumors that were on active chemotherapy or radiotherapy, or those with an advanced disease that could affect the short-term prognosis), and other immunodeficiencies. Furthermore, we recorded the number of COVID-19 vaccine doses received, the time since the last dose, and the vaccine brand (BNT162b2, mRNA-1273, or a combination). We also determined if the episode was COVID-19 reinfection and if it was nosocomially acquired as per CDC guidelines [15]. The minimum follow-up period was 30 days, and it extended until the end of the disease course (complete clinical recovery or death). Patients were treated for COVID-19 as per Ontario Science Table guidelines [16]. All transplant recipients were assessed in the hospital’s COVID care virtual clinic by a physician or nurse specializing in transplant care. All patients were considered high risk and eligible for monoclonal antibody therapy (sotrovimab) if they presented within 7 days of symptom onset and were not hypoxic.
Outcomes
The primary outcome evaluated was the need for supplemental oxygen within 30 days of COVID-19 diagnosis. This variable included both patients that needed to start oxygen therapy and those with oxygen at baseline that presented an increase in their requirements. Secondary outcomes included hospitalization >24 hours related to COVID-19, admission to the ICU, mechanical ventilation, and all-cause mortality. All the secondary outcomes were evaluated within 30 days of COVID-19 diagnosis. To estimate the effectiveness of the number of vaccines on the outcomes the population was stratified in 2 groups: patients with 3 or more vaccines, and patients with less than 3 vaccines. We compared the presence of outcomes by number of vaccines and by sotrovimab therapy. Patients with nosocomial acquisition of COVID-19 were excluded from the outcome analysis. Patients that received sotrovimab after the onset of hypoxia were also excluded from the effectiveness analysis of this treatment as it was outside guidelines.
Other clinical outcomes registered were the presence of COVID-19 pneumonia, the length of stay in hospital and ICU, and allograft rejection in the first 30 days. Rejection needed to be biopsy proven or clinically diagnosed and treated. Various complications after COVID-19 were recorded, including: acute kidney injury, cardiac and neurologic adverse events, bacterial superimposed pneumonia, COVID-19-associated pulmonary aspergillosis (CAPA), bloodstream infection, and others. We also recorded the administration of dexamethasone, remdesivir, tocilizumab, and baricitinib, and the changes in the maintenance immunosuppression especially calcineurin inhibitors (CNI) and antimetabolite drugs.
Statistical Analysis
Demographics and baseline characteristics were analyzed by the primary endpoint of oxygen requirement. Risk factors were estimated by univariate analysis using the Fisher’s exact test to compare categorical variables, and the Mann-Whitney U test (Wilcoxon rank-sum test) to compare continuous variables. P values <.05 were considered significant. We estimated the risk of each outcome based on the vaccination status of the patient and having received treatment with sotrovimab. For vaccination status, the risks were compared with ratios and differences (per 100 persons), and for sotrovimab, we calculated risk ratios and number needed to treat. We adjusted the P value of the association between the exposure (≥3 doses or <3 doses of the vaccine, and having sotrovimab or not) and the main outcomes (oxygen requirement and COVID-19-related hospitalization) using logistic regression. The covariates of the logistic regression model were selected based on risk factors that were significantly associated with the outcome (P < .05) on the univariate analysis. For this analysis, we considered the number of comorbidities for each patient rather than individual comorbidities. Statistical analyses were performed with Stata statistical software, version 15.1 (StataCorp, LLC, College Station, Texas, USA).
RESULTS
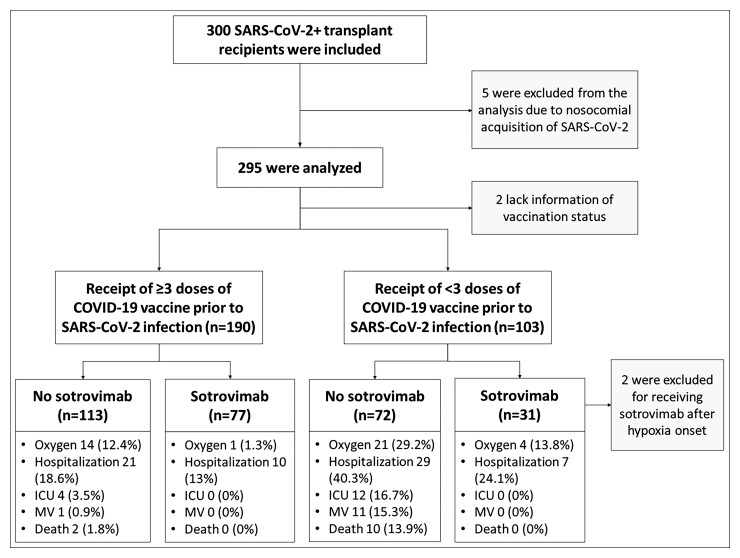
A total of 300 organ transplant recipients were included in the study and followed for at least 30 days from the COVID-19 diagnosis. Five patients were excluded from the outcome analysis due to nosocomial COVID-19 acquisition. Figure 1 shows the flow diagram of the study population, and the outcomes stratified by vaccination status and sotrovimab treatment.
Figure 1.
Flow diagram of the study population and outcomes stratified by vaccination status and sotrovimab treatment. Hospitalization indicates >24 hours COVID-19-related hospitalization, death indicates all-cause mortality. All the outcomes were evaluated within 30 days of COVID-19 diagnosis. Abbreviations: COVID-19, coronavirus disease 2019; ICU, intensive care unit; MV, mechanical ventilation; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
The demographic characteristics are shown in Table 1. Transplant types were kidney (42.7%), lung (20.7%), liver (15.3%), heart (9.0%), and kidney-pancreas (9.3%). The majority were on triple immunosuppression with prednisone, mycophenolate and a calcineurin inhibitor. Four (1.3%) patients had asymptomatic infection. Although vaccine was available for transplant patients since February 2021, 4% of patients remained unvaccinated. Seven of the 295 patients analyzed had a reinfection (2.4%).
Table 1.
Demographic and Clinical Characteristics of Transplant Recipients at Baseline by Vaccination Statusa
| Characteristics | Total (N = 300) | Patients With <3 Vaccines (N = 104) | Patients With ≥3 Vaccines (N = 194) | P Value b |
|---|---|---|---|---|
| Age, y (mean ± SD) | 53.9 ± 14.2 | 52.5 ± 15.1 | 54.8 ± 13.1 | .52 |
| Female sex, no. (%) | 129 (43%) | 43 (41.3%) | 86 (44.3%) | .63 |
| Type of transplant, no. (%) | ||||
| Kidney | 128 (42.7%) | 51 (49%) | 76 (39.2%) | .11 |
| Lung | 62 (20.7%) | 21 (20.2%) | 40 (20.6%) | 1.00 |
| Liver | 46 (15.3%) | 17 (16.3%) | 29 (14.9%) | .74 |
| Heart | 27 (9%) | 9 (8.7%) | 18 (9.3%) | 1.00 |
| Kidney-pancreas | 28 (9.3%) | 6 (5.8%) | 22 (11.3%) | .15 |
| Other combined transplantsa | 9 (3%) | 0 (0%) | 9 (4.6%) | .03 |
| Retransplant, no. (%) | 25 (8.4%) | 7 (6.7%) | 18 (9.3%) | .52 |
| Years since transplant (mean ± SD) | 7.3 ± 7.2 | 7.4 ± 7.8 | 7.3 ± 6.9 | .48 |
| Coexisting conditions, no. (%) | ||||
| Hypertension | 223 (74.3%) | 73 (70.2%) | 148 (76.3%) | .27 |
| Diabetes mellitus | 110 (36.7%) | 33 (31.7%) | 76 (39.2%) | .21 |
| BMI ≥ 30 | 55 (18.3%) | 21 (20.2%) | 33 (17.0%) | .53 |
| Coronary artery disease | 51 (17.1%) | 15 (14.4%) | 35 (18.1%) | .52 |
| Chronic cardiac failure | 21 (7%) | 6 (5.8%) | 14 (7.2%) | .81 |
| Chronic lung disease | 56 (18.7%) | 19 (18.3%) | 36 (18.6%) | 1.00 |
| Chronic kidney diseasea | 124 (41.3%) | 43 (41.3%) | 79 (40.7%) | 1.00 |
| Active systemic malignancya | 4 (1.3%) | 2 (1.9%) | 2 (1%) | .61 |
| Other immunodeficiencya | 3 (1%) | 1 (1%) | 2 (1%) | 1.00 |
| No. of comorbidities (mean ± SD) | 2.2 ± 1.3 | 2 ± 1.4 | 2.2 ± 1.3 | .40 |
| Immunosuppressant, no. (%) | ||||
| Prednisone | 253 (84.6%) | 87 (84.5%) | 165 (85.1%) | 1.00 |
| Daily dose, mg (median, IQR) | 5 (5 to 7.5) | 5 (5 to 10) | 5 (5 to 5) | .11 |
| Tacrolimus | 242 (80.7%) | 82 (78.8%) | 158 (81.4%) | .65 |
| Last level, ng/mL (mean ± SD) | 8.2 ± 3.8 | 8.6 ± 4.9 | 8 ± 3 | .94 |
| Cyclosporine | 49 (16.3%) | 17 (16.3%) | 32 (16.5%) | 1.00 |
| Mycophenolate | 239 (79.7%) | 84 (80.8%) | 154 (79.4%) | .76 |
| Daily dose, mg (median, IQR) | 1080 (720 to 1440) | 1080 (720 to 1440) | 720 (720 to 1440) | .41 |
| Azathioprine | 25 (8.3%) | 8 (7.8%) | 16 (9.4%) | 1.00 |
| Sirolimus | 9 (3%) | 4 (3.8%) | 5 (2.6%) | .72 |
| Rejection last 3 months – no. (%) | 5 (1.7%) | 3 (2.9%) | 2 (1%) | .35 |
| ATG last 3 months – no. (%) | 2 (0.7%) | 2 (1.9%) | 0 (0%) | .12 |
| Basiliximab last 3 months – no. (%) | 8 (2.7%) | 5 (4.8%) | 3 (1.6%) | .13 |
| Rituximab last 3 months – no. (%) | 1 (0.3%) | 1 (1%) | 0 (0%) | .35 |
| Time since last COVID-19 vaccine – days (median, IQR) | 104 (59 to 152) | 194 (124 to 235) | 91 (46 to 111) | <.001 |
| Vaccine brand, no. (%) | ||||
| BNT162b2 (Pfizer–BioNTech) | 146 (48.66%) | 54 (51.9%) | 92 (66.2%) | .11 |
| mRNA-1273 (Moderna) | 45 (15%) | 14 (13.5%) | 31 (15.9%) | .86 |
| Mix | 19 (6.3%) | 2 (1.9%) | 16 (11.5%) | .04 |
| SARS-CoV-2 reinfection – no. (%) | 7 (2.3%) | 1 (1%) | 6 (3.2%) | .43 |
| Sotrovimab treatment – no. (%) | 109 (36.3%) | 32 (30.7%) | 78 (40.2%) | .13 |
Abbreviations: ATG, anti-thymocyte globulin; BMI, body mass index; COVID-19, coronavirus disease 2019; GFR, glomerular filtration rate; IQR, interquartile range; PTLD, post-transplant lymphoproliferative disorder; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; SD, standard deviation.
Vaccine information not known for 2 patients. Other combined transplants include: kidney-liver, lung-liver, kidney-heart, and lung-heart transplant. Chronic kidney disease denotes GFR < 60 ml/min/m2. Other immunodeficiency includes 2 HIV and 1 asplenic patient. Active systemic malignancy includes PTLD, lung cancer and intrahepatic cholangiocarcinoma.
Continuous variables P-value estimated using Mann Whitney U-test (Wilcoxon rank-sum). Categorical variables P-value estimated using Fisher’s exact test.
Outcomes
During the follow-up period, a total of 71 patients (24.1%) were hospitalized, 44 (14.9%) required supplemental oxygen, 19 (6.5%) were admitted to the ICU, 15 (5.1%) required mechanical ventilation (MV), and 13 (4.4%) died. Significant patient characteristics associated with oxygen requirement are shown in Table 2. On univariate analysis, age and multiple comorbidities (diabetes mellitus, coronary artery disease, chronic cardiac failure, chronic lung disease, chronic kidney disease, active malignancy) were significantly associated with the need for supplemental oxygen. Liver transplant was associated with less risk of needing supplemental oxygen and hospitalization than other organ types (P = .025). There were no differences between different immunosuppressive therapies. Multivariate analysis showed that age (odds ratio [OR] 1.07 (95% confidence interval [CI] 1.03 to 1.12), P < .001), and number of comorbidities (OR 1.83 (95% CI 1.30 to 2.58), P < .001) were risk factors for oxygen requirement. However, having a liver transplant (OR 0.10 (95% CI .01 to .79), P = .029), receipt of ≥3 doses of vaccine, and early therapy with sotrovimab were independently protective factors (below and Table 3). Patient characteristics by sotrovimab use are shown in the Supplementary material (Supplementary Table 1).
Table 2.
Variables Associated With Oxygen Requirement
| Characteristics | Patient Without Oxygen (N = 251)a | Patients With Oxygen (N = 44)a | P Valueb |
|---|---|---|---|
| Age, y (mean ± SD) | 52.7 ± 13.7 | 63.4 ± 11.4 | <.001 |
| Type of transplant, no. (%) | |||
| Liver | 42 (16.7%) | 2 (4.5%) | .025 |
| Coexisting conditions, no. (%) | |||
| Diabetes mellitus | 87 (34.7%) | 23 (52.3%) | .043 |
| Coronary artery disease | 34 (13.5%) | 17 (38.6%) | <.001 |
| Chronic cardiac failure | 11 (4.4%) | 10 (22.7%) | <.001 |
| Chronic lung disease | 41 (16.3%) | 15 (34.1%) | .011 |
| Active systemic malignancyc | 1 (0.40%) | 3 (6.8%) | .011 |
| No. of comorbidities (mean ± SD) | 1.9 ± 1.2 | 3.1 ± 1.5 | <.001 |
| Time since last COVID-19 vaccine – days (median, IQR) | 100 (59 to 134) | 145 (84 to 224) | .001 |
Abbreviations: COVID-19, coronavirus disease 2019; IQR, interquartile range; PTLD, post-transplant lymphoproliferative disorder; SD, standard deviation.
Five patients were excluded from the oxygen analysis due to nosocomial COVID-19 acquisition.
Continuous variables P-value estimated using Mann-Whitney U test (Wilcoxon rank-sum). Categorical variables P-value estimated using Fisher exact test.
Active systemic malignancy includes PTLD, lung cancer and intrahepatic cholangiocarcinoma.
Table 3.
Outcomes Through Day 30
| By Vaccination Status | |||||
|---|---|---|---|---|---|
| End Points | ≥3 Vaccines (N = 190) | <3 Vaccines (N = 103) | Risk Ratio (95% CI) | Risk Diff. (95% CI)a | Adjusted P Valueb |
| Primary end point | |||||
| Oxygen requirement by day 30, no. (%) | 15 (7.9%) | 27 (26.2%) | 0.30 (.17 to .54) | −18.3 (−28 to −9.4) | <.001 |
| Secondary end points | |||||
| COVID-19-related hospitalization >24 h, no. (%) | 31 (16.3%) | 38 (36.9%) | 0.44 (.29 to .67) | −20.5 (−31.2 to −10.1) | <.001 |
| COVID-19-related ICU admission, no. (%) | 4 (2.1%) | 13 (12.7%) | 0.14 (.05 to .46) | −10.6 (−18.6 to −4.6) | |
| Mechanical ventilation requirement, no. (%) | 1 (0.5%) | 12 (11.7%) | 0.04 (.01 to .31) | −11.1 (−18.8 to −5.7) | |
| All-cause mortality, no. (%) | 2 (1.1%) | 10 (9.7%) | 0.10 (.02 to .46) | −8.7 (−15.9 to −3.5) | |
| By Sotrovimab treatment | |||||
| End points | Sotrovimab (N = 106) | No sotrovimab (N = 187) |
Risk ratio (95% CI) | NNT (95% CI)a | Adjusted P valueb |
| Primary end point | |||||
| Oxygen requirement, no. (%) | 5 (4.7%) | 37 (19.8%) | 0.24 (.10 to .59) | 6.6 (4.6 to 13.7) | .026 |
| Secondary end points | |||||
| COVID-19-related hospitalization >24 h, no. (%) | 17 (16%) | 52 (27.8%) | 0.58 (.35 to .94) | 8.5 (4.8 to 59.1) | .33 |
| COVID-19-related ICU admission, no. (%) | … | 18 (9.6%) | … | 10.4 (6.8 to 21.3) | |
| Mechanical ventilation requirement, no. (%) | … | 14 (7.5%) | … | 13.4 (8.2 to 34.5) | |
| All-cause mortality, no. (%) | … | 13 (7%) | … | 14.4 (8.7 to 40.9) | |
Abbreviations: CI, confidence interval; COVID-19, coronavirus disease 2019; ICU, intensive care unit; NNT, number needed to treat to prevent 1 event.
Risk difference expressed on number of events per 100 patients that received ≥3 mRNA vaccine doses. Newcombe confidence interval used for both risk difference and number needed to treat.
Adjusted P value estimated with multivariate logistic regression. Stratification factors included in both the oxygen requirement and the hospitalization models were: receipt 3 or more mRNA COVID-19 vaccines, sotrovimab treatment, age, liver transplant, and number of comorbidities.
Impact of Vaccination
A total of 190 patients (65.3%) had received three or more doses of COVID-19 vaccine prior to SARS-CoV-2 infection. Ninety patients (30.2%) received two doses. Supplemental oxygen was needed in 15/190 (7.9%) patients with ≥3 doses of mRNA vaccine and 27/103 (26.2%) in those with <3 doses: risk ratio (RR) 0.30 (95% CI, .17 to .54). Regarding hospitalization, 31/190 (16.3%) of the patients with ≥3 vaccine doses and 38/103 (36.9%) of those with <3 doses were admitted: RR 0.44 (95% CI, .29 to .67). The effect of ≥3 doses of mRNA vaccine in the oxygen requirement and hospitalization rate persisted after adjusting by age, having a liver transplant, number of comorbidities, and sotrovimab treatment. Other secondary outcomes (ICU admission, MV requirement, and all-cause mortality) were not adjusted due to the lack of events. Table 3 summarizes the association measures between vaccination status and primary and secondary outcomes.
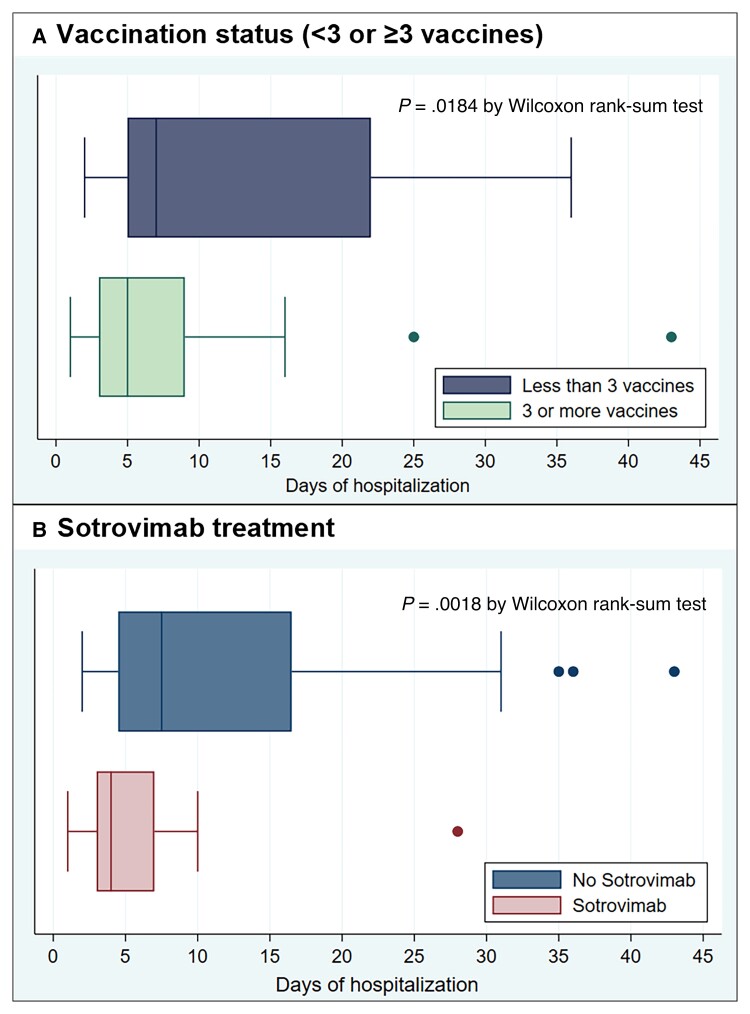
There were no differences in the outcomes when comparing the patients that received the BNT162b2 vaccine with those that received the mRNA-1273 vaccine. Although the time since the last dose of mRNA vaccine was significantly associated with a lower rate of oxygen requirement and hospitalization (Table 2), after adjusting by the number of doses received, this association was no longer significant. In patients with ≥3 vaccine doses, median hospital stay was 5 days (IQR, 3–9), and 7 days in those <3 doses (IQR, 5–22), P = .018 (Figure 2A). Other adverse outcomes such as acute kidney injury, cardiac and neurologic events as well as bacterial infections were more frequent in those who had received <3 doses of vaccine (Supplementary Table 2).
Figure 2.
Time of admission by vaccination status and sotrovimab treatment. Shown are box plots of days of COVID-19-related hospitalization by day 30, according to whether the patient has received ≥3 or <3 vaccine doses (A), or was treated with sotrovimab or not (B). In each box plot, the vertical line represents the median value, with the left and the right of the box indicating the 25th percentile and 75th percentile, respectively; the whiskers indicate values that are within 1.5 times the interquartile range. Circles represents outside values (individual patients with hospitalization time far from the rest). Abbreviation: COVID-19, coronavirus disease 2019.
Impact of Sotrovimab
A total of 108 patients (36.6%) received sotrovimab within 7 days of symptom onset. Two patients were excluded from the analysis due to sotrovimab administration after the onset of hypoxia. Oxygen requirement occurred in 5/106 (4.7%) patients that received sotrovimab and 37/187 (19.8%) of those that did not; RR 0.24 (95% CI, .1 to .59), number needed to treat (NNT) to prevent one patient needing supplemental oxygen was 6.64 (95% CI, 4.56 to 13.66). Hospitalization occurred in 17/106 (16%) patients that received early sotrovimab and 52/187 (27.8%) that did not: RR 0.58 (95% CI, .35 to .94), NNT to prevent one hospitalization was 8.5 (95% CI, 4.83 to 59.1). No patient in the sotrovimab group was admitted to the ICU, required MV or died by day 30 of follow-up. The association measures between sotrovimab treatment and the different outcomes are summarized in Table 3. The effect of sotrovimab on the oxygen requirement persisted after adjusting by age, type of transplant, number of vaccines, and number comorbidities. On the other hand, the association with the admission rate was not statistically significant in the multivariate analysis (Table 3). However, sotrovimab use was associated with a shorter hospitalization time: 4 days in the sotrovimab group (interquartile range [IQR], 3 to 7), and 7 days in the untreated group (IQR, 5 to 16), P = .002 (Figure 2B). COVID-19 pneumonia was more frequently diagnosed in those that did not receive sotrovimab and there was greater use of dexamethasone and remdesivir in this group (Supplementary Table 3).
In exploratory analyses, the group that had received less than 3 doses of vaccine and also did not receive sotrovimab (n = 72) tended to have the poorest outcomes with oxygen requirement in 21/72 (29.2%) and death in 10/72 (13.9%) (Figure 1 and Supplementary Figure 1). The group that had ≥3 doses of vaccine and received early sotrovimab had the best outcomes with only 1/77 (1.3%) requiring supplemental oxygen and no deaths.
DISCUSSION
We conducted a prospective observational study in a cohort of organ transplant recipients during the Omicron wave. We found that advanced age and multiple comorbidities remain important factors in determining risk of severe disease. However, we also found that having at least 3 doses of mRNA vaccine and receiving sotrovimab early in the course of infection significantly reduced the risk of severe disease as measured by the need for supplemental oxygen.
Overall, during the Omicron wave, rates of hospitalization, ICU, and mortality in our study were lower than previously reported with non-Omicron variants [3, 17, 18]. The milder outcomes are consistent with reports in the general population [12]. However, in the transplant setting, this may also be a function of prior vaccination and early monoclonal antibody. Maintenance immunosuppression analyzed as individual drugs was not a significant factor associated with disease severity in the overall cohort. This is consistent with other studies [17, 19]. However, lower levels and doses of maintenance immunosuppression likely contribute to the lower risk of severe disease in liver transplant recipients. Immunosuppression was reduced in many patients over the course of illness especially in those that had more severe disease.
Three doses of vaccine show a greater neutralizing antibody response to wild-type and variants of SARS-CoV-2 [6, 20]. However, the neutralizing antibody response to Omicron variant is poor and this is consistent with the number of infections seen in the transplant population despite vaccination [21]. Still, despite infection, we show that three doses of vaccine significantly reduced the risk of needing supplemental oxygen and hospitalization. Having at least three doses of vaccine was also associated with a reduced length of stay in patients that were hospitalized. This suggests that factors other than neutralizing antibodies, namely, T-cell immunity, are important in reducing severity of disease especially in variants showing antibody escape. Interestingly, 7 patients who were infected with non-Omicron strains in the past were re-infected suggesting that natural immunity is also not necessarily protective against infection. It is likely that antibodies produced by non-Omicron SARS-CoV-2 infection do not cross-neutralize omicron or that waning of antibody occurs over time. Only 8 patients had received their fourth dose of vaccine (recommended as a booster); therefore, no conclusion can be made regarding its additional benefit. Of note, in the univariate analysis, a longer time from last dose of vaccine was associated with increased disease severity suggesting waning immunity.
We also found that sotrovimab when given within the first 7 days of symptom onset was successful in reducing the need for supplemental oxygen and hospitalization. This is consistent with studies of monoclonal antibodies in the general population [22]. Moreover, in our study it reduced burden on the healthcare system by reducing duration of hospitalization and need for other COVID-19 therapies. A benefit was noted despite the fact that 4 asymptomatic patients were included in the cohort, none of whom received sotrovimab.
We did not take vaccination status or antibody levels into consideration when prescribing sotrovimab. Our protocol was to offer sotrovimab to all transplant recipients within the first 7 days of symptoms. Some patients did not receive sotrovimab if they had >7 days of symptoms, lack of an infusion center in their area, were not able to access the infusion center, or refused to have therapy. It is remarkable that no patient that received sotrovimab needed ICU care. This is despite a greater number of lung transplant recipients that were in the untreated group.
Strengths of our study include a large sample size with prospective cohort design. The results can lead to actionable recommendations. Our study has some limitations. The majority of patients in the study were diagnosed and treated based on rapid antigen testing. This was due to unavailability of polymerase chain reaction (PCR) test appointments and slow turnaround for results during the Omicron wave from mid-December to January. However, all patients had compatible symptoms and those that were eventually hospitalized were confirmed using SARS-CoV-2 PCR. Not all patients had sequencing to confirm infection with Omicron variant. However, >90% of isolates submitted to public health laboratory by 15 December 2021 and >99% of isolates sequenced by 31 December 2021 were Omicron variant BA.1. The study cohort was infected prior to the approval of nirmatrelvir/ritonavir in Canada; therefore no patient was treated with outpatient antivirals, and no patient received other outpatient therapies including fluvoxamine or budesonide.
In summary, we provide evidence of real-world effectiveness of vaccine and early monoclonal antibody therapy in transplant patients infected with SARS-CoV-2 during the Omicron wave. In our study, sotrovimab treatment was associated with a significant reduction in the supplementary oxygen requirement rate even after adjusting for vaccination status and comorbidities. We suggest that full vaccination for a transplant recipient be defined by at least three doses of vaccine. In addition, transplant recipients should be counseled to report their symptoms and get tested rapidly so that early monoclonal antibody therapy can be instituted.
Supplementary Material
Contributor Information
Javier T Solera, Ajmera Transplant Centre, University Health Network, Toronto, Canada.
Berta G Árbol, Ajmera Transplant Centre, University Health Network, Toronto, Canada.
Abdullah Alshahrani, Ajmera Transplant Centre, University Health Network, Toronto, Canada.
Ilona Bahinskaya, Ajmera Transplant Centre, University Health Network, Toronto, Canada.
Nikki Marks, Ajmera Transplant Centre, University Health Network, Toronto, Canada.
Atul Humar, Ajmera Transplant Centre, University Health Network, Toronto, Canada.
Deepali Kumar, Ajmera Transplant Centre, University Health Network, Toronto, Canada.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Note
Potential conflicts of interest. D. K. has received research grants from Roche, GSK (paid to institution) and advisory fees from Roche, GSK, Merck, Astellas, Sanofi, Exevir (payments to author), and payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Astellas and Meducom (payments to author) D. K. also reports a leadership or fiduciary role on the Board of Directors for the American Society of Transplantation. A. H. has received clinical trials grant from Merck (paid to institution) and advisory fees from Merck (paid to author). All other authors report no potential conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Kates OS, Haydel BM, Florman SS, et al. COVID-19 in solid organ transplant: a multi-center cohort study. Clin Infect Dis 2021; 73:e4090–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Pereira MR, Mohan S, Cohen DJ, et al. COVID-19 in solid organ transplant recipients: initial report from the US epicenter. Am J Transplant 2020; 20:1800–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Marinelli T, Ferreira VH, Ierullo M, et al. Prospective clinical, virologic, and immunologic assessment of COVID-19 in transplant recipients. Transplantation 2021; 105:2175–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Embi PJ, Levy ME, Naleway AL, et al. Effectiveness of 2-dose vaccination with mRNA COVID-19 vaccines against COVID-19-associated hospitalizations among immunocompromised adults - nine states, January–September 2021. MMWR Morb Mortal Wkly Rep 2021; 70:1553–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Qin CX, Moore LW, Anjan S, et al. Risk of breakthrough SARS-CoV-2 infections in adult transplant recipients. Transplantation 2021; 105:e265–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hall VG, Ferreira VH, Ku T, et al. Randomized trial of a third dose of mRNA-1273 vaccine in transplant recipients. N Engl J Med 2021; 385:1244–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Coronavirus (COVID-19) update: FDA authorizes additional vaccine dose for certain immunocompromised individuals. Available at: https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-additional-vaccine-dose-certain-immunocompromised. Accessed 18 March 2022.
- 8. Yetmar ZA, Beam E, O'Horo JC, et al. Monoclonal antibody therapy for COVID-19 in solid organ transplant recipients. Open Forum Infect Dis 2021; 8:ofab255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Vellas C, Del Bello A, Gaube G, et al. Impact of casirivimab-imdevimab on severe acute respiratory syndrome coronavirus 2 delta variant nasopharyngeal virus load and spike Quasispecies. Open Forum Infect Dis 2022; 9:ofac093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Planas D, Saunders N, Maes P, et al. Considerable escape of SARS-CoV-2 Omicron to antibody neutralization. Nature 2022; 602:671–5. [DOI] [PubMed] [Google Scholar]
- 11. Cameroni E, Bowen JE, Rosen LE, et al. Broadly neutralizing antibodies overcome SARS-CoV-2 Omicron antigenic shift. Nature 2022; 602:664–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nyberg T, Ferguson NM, Nash SG, et al. Comparative analysis of the risks of hospitalisation and death associated with SARS-CoV-2 omicron (B.1.1.529) and delta (B.1.617.2) variants in England: a cohort study. Lancet 2022; 399:1303–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kumar D, Ferreira VH, Blumberg E, et al. A 5-year prospective multicenter evaluation of influenza infection in transplant recipients. Clin Infect Dis 2018; 67:1322–9. [DOI] [PubMed] [Google Scholar]
- 14. SARS-CoV-2 Whole Genome Sequencing in Ontario, March 8, 2022. Available at: https://www.publichealthontario.ca/-/media/Documents/nCoV/epi/covid-19-sars-cov2-whole-genome-sequencing-epi-summary.pdf? sc_lang=en. Accessed 18 March 2022.
- 15. Coronavirus Disease 2019 (COVID-19) 2021 Case Definition. Available at: https://ndc.services.cdc.gov/case-definitions/coronavirus-disease-2019-2021/. Accessed 18 March 2022.
- 16. Ontario COVID-19 Drugs and Biologics Clinical Practice Guidelines Working Group . Clinical practice guideline summary: recommended drugs and biologics in adult patients with COVID-19. Ontario COVID-19 Science Advisory Table. 2022; Version 7.0. doi:10.47326/ocsat.cpg.2022.7.0.
- 17. Kates OS, Haydel BM, Florman SS, et al. Coronavirus disease 2019 in solid organ transplant: a multicenter cohort study. Clin Infect Dis 2021; 73:e4090–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Pereira MR, Mohan S, Cohen DJ, et al. COVID-19 in solid organ transplant recipients: initial report from the US epicenter. Am J Transplant 2020; 20:1800–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Raja MA, Mendoza MA, Villavicencio A, et al. COVID-19 in solid organ transplant recipients: a systematic review and meta-analysis of current literature. Transplant Rev (Orlando) 2021; 35:100588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kumar D, Ferreira VH, Hall VG, et al. Neutralization of SARS-CoV-2 variants in transplant recipients after two and three doses of mRNA-1273 vaccine: secondary analysis of a randomized trial. Ann Intern Med 2022; 175:226–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kumar D, Hu Q, Samson R, et al. Neutralization against Omicron variant in transplant recipients after three-doses of mRNA vaccine. Am J Transplant 2022. 10.1111/ajt.17020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gupta A, Gonzalez-Rojas Y, Juarez E, et al. Effect of sotrovimab on hospitalization or death among high-risk patients with mild to moderate COVID-19: a randomized clinical trial. JAMA 2022; 327:1236–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.