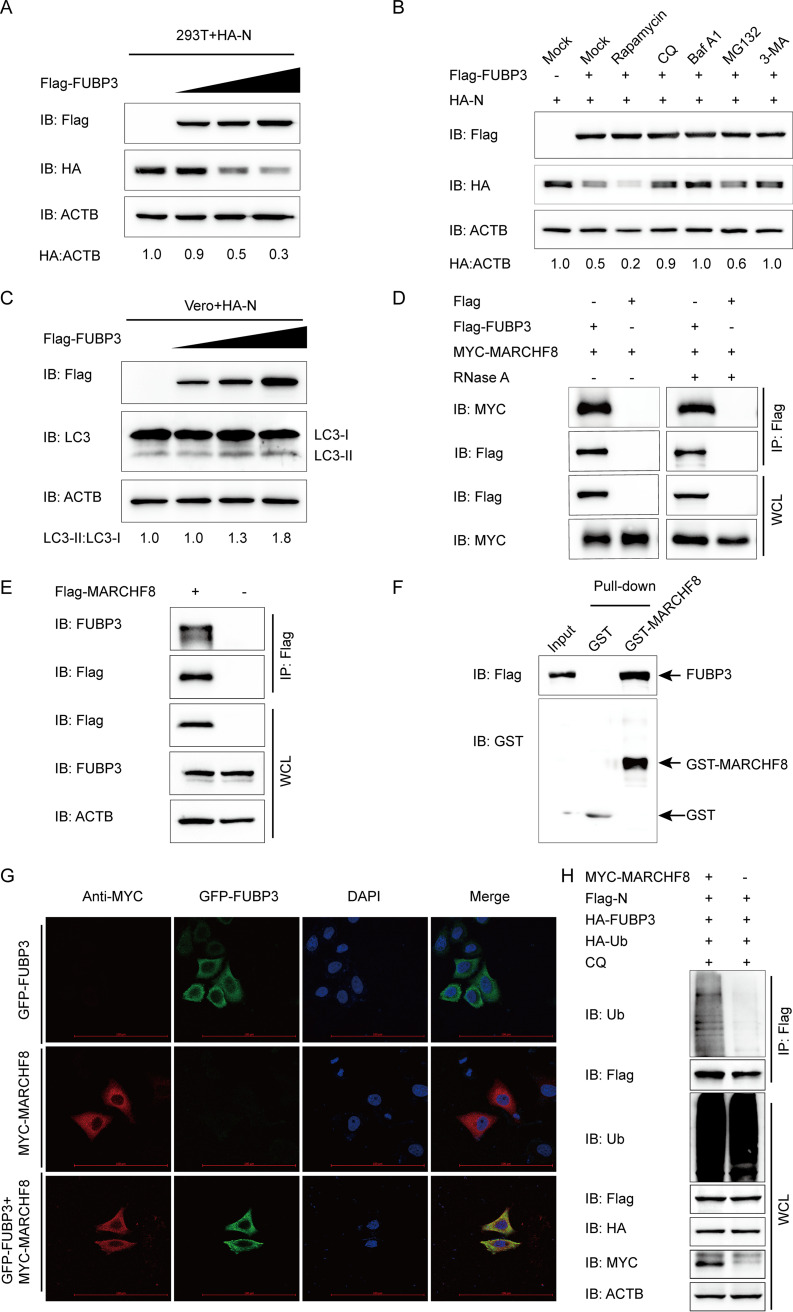
FIG 4.
FUBP3 can enhance autophagic degradation of N by recruiting MARCHF8 to ubiquitinate the N protein. (A) The HA-N expression vector was transfected into HEK 293T cells, and subsequently, the Flag-FUBP3 expression vector (wedge) at increasing doses was also transfected into the cells. WB was conducted to analyze the cell lysates. ACTB functioned as a sample loading control. (B) The Flag-FUBP3 and HA-N expression vectors were transfected into the HEK 293T cells for 24 h, followed by treatment with Rapamycin (12.5 μM), CQ (50 μM), Baf A1 (50 μM), MG132 (5 μM), or 3-MA (1 mM) for 9 h. WB was conducted to analyze the cell lysates. (C) The HA-N expression vector was transfected into the HEK 293T cells along with the Flag-FUBP3 expression vector (wedge) at high doses. WB was conducted to analyze the cell lysates. (D) The plasmids that encoded MYC-MARCHF8 and Flag-FUBP3 were transfected into HEK 293T cells, and later, a Co-IP assay was carried out with the use of anti-Flag-bound beads, followed by WB analysis. (E) The plasmid that encoded Flag-MARCHF8 was transfected into HEK 293T cells for 24 h. Subsequently, a Co-IP assay was carried out with an anti-Flag antibody. (F) The expression of GST-MARCHF8 and FUBP3 was induced in the bacterial strain BL21(DE3), with the relationship between MARCHF8 and FUBP3 being analyzed by performing a GST pulldown assay. (G) Followed by incubation of the cells with anti-MYC MAb, FUBP3-GFP and MARCHF8-MYC were cotransfected into HeLa cells for a whole day. The colocalization of FUBP3 and MARCHF8 was found by confocal IF microscopy; scale bars: 100 μm. (H) The plasmids that encoded MYC-MARCHF8, Flag-N, HA-FUBP3, and HA-Ub were cotransfected into HEK 293T cells for 24 h, followed by treatment with CQ (50 μM) for 9 h. A Co-IP assay was carried out with an anti-Flag antibody. In the meanwhile, a WB assay was conducted to analyze the cell lysates with an anti-ubiquitin (Ub) antibody.