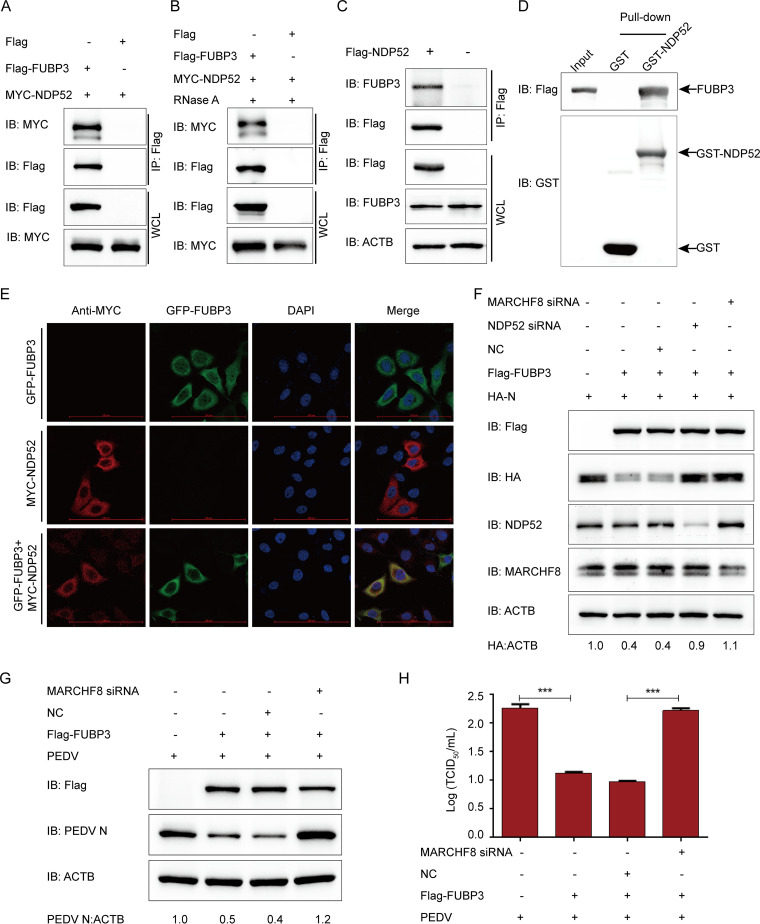
FIG 5.
FUBP3 caused N protein degradation via the MARCHF8-NDP52-autophagosome pathway. (A) Plasmids that encoded MYC-NDP52 and Flag-FUBP3 were cotransfected into HEK 293T cells, then a Co-IP assay was conducted based on anti-Flag-bound beads, and a WB was conducted for the analysis. (B) The HEK 293T cells cotransfected with Flag-FUBP3 and MYC-NDP52 for 24 h were harvested. RNase was used for incubating the lysates, and the correlation between FUBP3 and NDP52 was analyzed by Co-IP assays. (C) The plasmids that encoded Flag-NDP52 were transfected into HEK 293T cells for a day. Co-IP assays were conducted using an anti-Flag antibody. (D) The expression of GST-NDP52 and FUBP3 was induced in the bacterial strain BL21(DE3). The relationship between FUBP3 and NDP52 was analyzed by performing a GST pulldown analysis. (E) HeLa cells were cotransfected with FUBP3-GFP and NDP52-MYC for 24 h based on the incubation with anti-MYC MAb. The colocalization of FUBP3 and NDP52 was found using confocal IF microscopy; scale bars: 100 μm. (F) NDP52 siRNA or MARCHF8 siRNA was transfected into HEK 293T cells with plasmids that encoded HA-N and Flag-FUBP3. WB was performed for analysis. (G and H) Vero cells were transfected with the plasmids encoding Flag-FUBP3 and MARCHF8 siRNA for 24 h, and subsequently, PEDV (MOI = 0.01) was used to infect cells. Western blot and TCID50 were analyzed at 20 h postinfection.