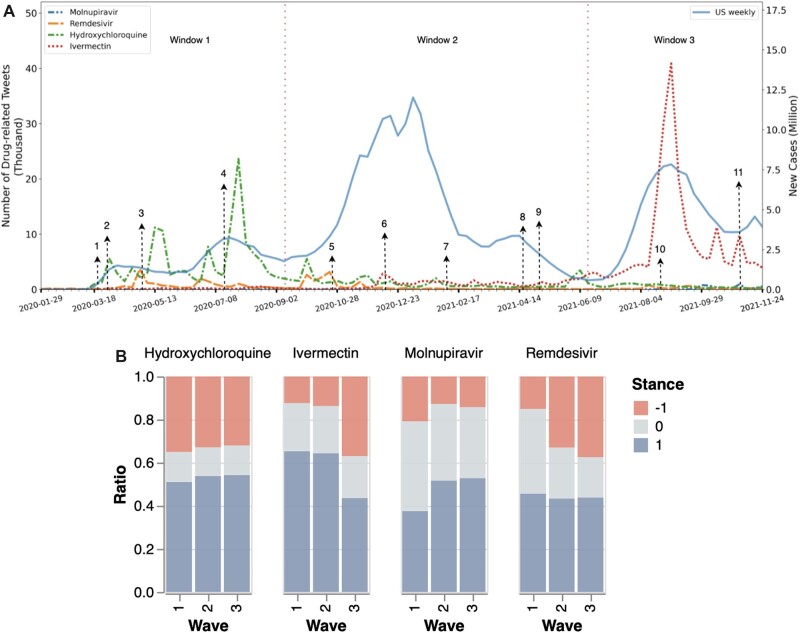
Figure 3.
(A) The trends of (1) the number of tweets that mentioned COVID-19-related drugs: Hydroxychloroquine, Ivermectin, Molnupiravir, Remdesivir, and (2) weekly COVID-19 case counts (stepped line) in the United States. Wave boundaries are noted by dashed vertical lines. Major drug events are noted by numbers: (1) March 19, 2020: Trump declared Hydroxychloroquine a game-changer; (2) March 28, 2020: FDA approved a EUA to use Hydroxychloroquine for certain hospitalized patients; (3) May 1, 2020: FDA approved a EUA to use Remdesivir for severe patients; (4) July 15, 2020: FDA cautioned against the use of Hydroxychloroquine; (5) October 22, 2020: FDA approved Remdesivir for conditional use; (6) December 10, 2020: FDA cautioned against Ivermectin; (7) February 4, 2021: Merck cautioned against Ivermectin; (8) April 17, 2021: FDA clarified that Remdesivir was not approved; (9) May 1, 2021: FDA recalled a batch of Remdesivir vials, (10) August 21, 2021: FDA denounced Ivermectin as a COVID-19 treatment following an increase in overdoses; (11) November 4, 2021 Britain authorized Molnupiravir for COVID-19 treatment. (B) Distribution of percentage of tweets with positive (1, blue), neutral (0, gray), and negative (−1, red) stances for each drug. COVID-19: coronavirus disease 2019; EUA: Emergency Use Authorization FDA: US Food and Drug Administration.