Abstract
Introduction
Anti-interferon-γ (IFNγ) autoantibodies have been associated with disseminated mycobacterial infections, mostly in patients from Southeast Asia.
Purpose
We studied an American-born, Caucasian female with M. avium complex infection of the subglottic mucosa and brain for underlying etiologies of infection.
Methods
Plasma was screened for anticytokine autoantibodies using a Luminex-based approach. The ability of patient plasma to block IFNγ-induced STAT1 phosphorylation in normal blood cells was evaluated by flow cytometry with intracellular staining. Plasma inhibition of IFNγ production and IFNγ-induced cytokines in normal and patient blood cells washed of autologous plasma was also evaluated.
Results
Patient plasma contained high-titer IgG anti-IFNγ autoantibodies, primarily of the IgG1 subclass. Patient but not control plasma prevented IFNγ-induced STAT1 phosphorylation and expression of the IFNγ-inducible cytokines tumor necrosis factor (TNF) α and interleukin (IL)-12 in normal blood cells. Patient blood cells washed free of autologous plasma demonstrated normal IFNγ production and response.
Conclusions
Disseminated nontuberculous mycobacterial infections should always prompt immune evaluation. This first case of disseminated nontuberculous mycobacterial infection and anti-IFNγ autoantibodies in an American-born Caucasian suggests that anti-cytokine autoantibodies are not racially or regionally restricted.
Keywords: Anticytokine autoantibodies, interferon-gamma (IFNγ), nontuberculous mycobacteria, intracranial infection
Introduction
Abnormalities in the IL-12/IFNγ, and TNFα pathways are well-established causes of disseminated nontuberculous mycobacterial infections (DNTM). Patients from Thailand and Taiwan were recently found to have nontuberculous mycobacterial (NTM) and other opportunistic infections due to IFNγ autoantibodies [1]. To date, high titer neutralizing autoantibodies implicated in mycobacterial disease have been overwhelmingly reported in Asian-born Asians [1–5], suggesting involvement of both genetic and environmental factors. Here we describe an American-born Caucasian woman with no history of travel outside the United States, who presented with extrapulmonary Mycobacterium avium complex infection and high-titer neutralizing anti-IFNγ autoantibodies.
Case
A 39-year-old Caucasian woman with a history of asthma and tobacco use presented with shortness of breath in March 2010. Chest CT revealed a lingular mass with mediastinal and cervical lymphadenopathy. Twice in the previous month she was treated for asthma exacerbations with short courses of oral corticosteroids. Biopsy of her lingula and mediastinal lymph node revealed necrotizing granulomata with acid-fast bacilli. She started isoniazid, rifampin, ethambutol, and pyrazinamide empirically for tuberculosis. Over the next month she developed subglottic stenosis with stridor and respiratory failure leading to intubation and tracheostomy. Biopsies of the subglottic area and previous lingular biopsy both grew Mycobacterium avium complex (MAC). Therapy was changed to rifampin, ethambutol, and azithromycin, however she had trouble obtaining medications for 2 months. Dexamethasone was given for subglottic stenosis, ranging from 18 to 32 mg daily until March 2011.
After 8 months of inconsistent antimycobacterial therapy without systemic corticosteroids, she developed seizures, left-sided weakness with headaches, fevers, weight loss and night sweats. A right frontal lobe lesion with edema was seen on Magnetic Resonance Imaging (MRI) (Fig. 1). MAC grew from brain and meningeal specimens. Antimycobacterials were continued.
Fig. 1.
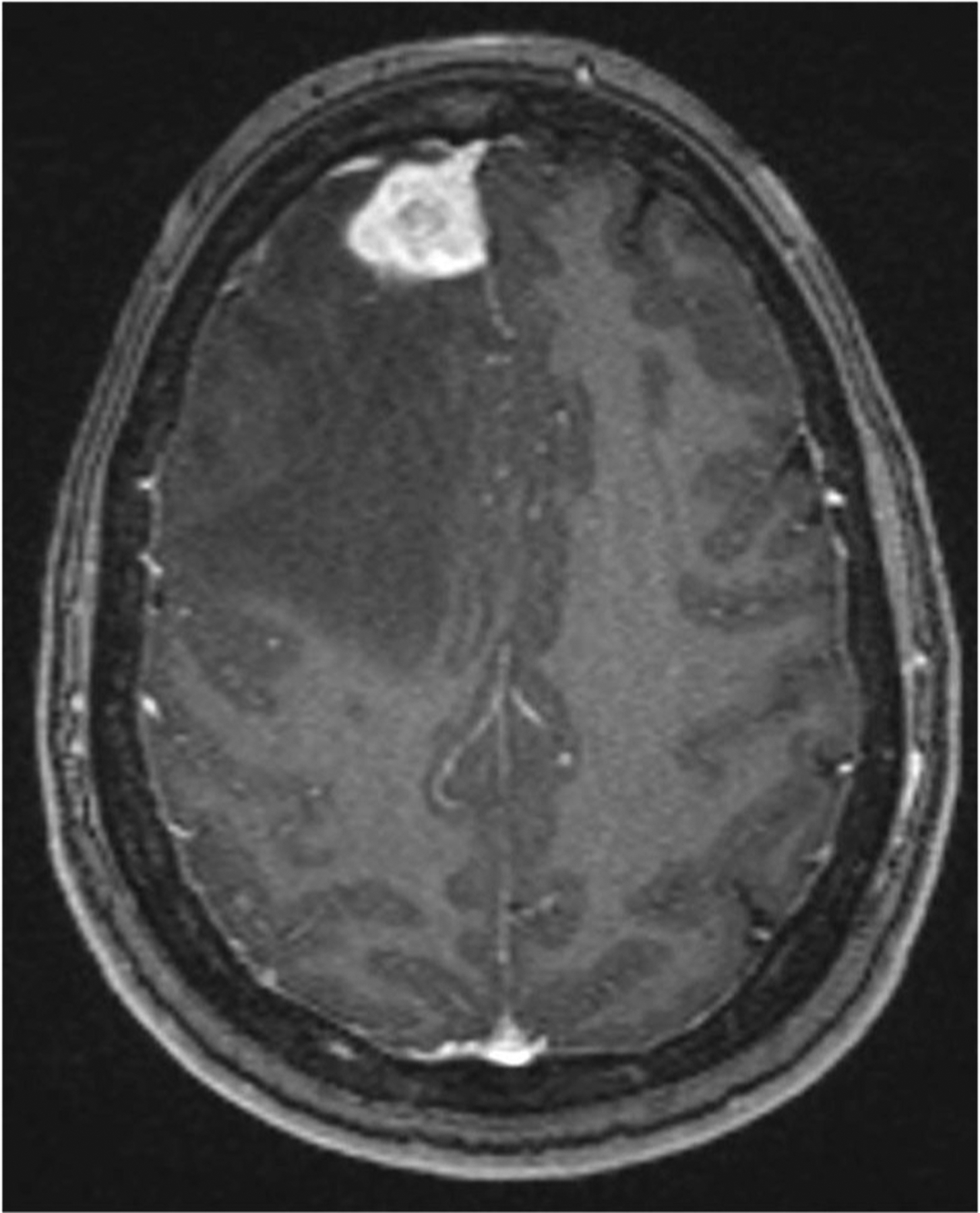
Axial T1 weighted post-contrast magnetic resonance imaging of the brain reveals right frontal parenchymal enhancing mass with internal areas of T1 signal, surrounded by marked vasogenic edema causing local mass effect and leftward midline shift
Seven months later, seizures recurred with headaches and MRI showed increased frontal subgaleal enhancement. Surgical resection of the infected bone revealed ongoing infection with MAC upon culture that remained sensitive to macrolides (clarithromycin MIC of 1). Repeated HIV testing was negative and CD4 counts in normal range.
Given her multifocal disease and poor response to therapy she was referred to the NIH for immunologic work-up.
Methods
Clinical Samples
Patient samples were collected under NIAID IRB-approved protocol (93-I-0119). Normal controls were obtained through the NIH Blood Bank under appropriate protocols. Whole blood was subjected to density gradient centrifugation to separate plasma and peripheral blood mononuclear cell (PBMC) fractions. Total IgG was purified from patient and normal plasma on protein G columns (Ab SpinTrap, GE Healthcare) per manufacturer’s instructions.
Determination of Anticytokine Autoantibodies
Patient and normal plasma were screened for anticytokine autoantibodies using a particle-based approach as previously described [6]. Anti-IFNγ-specific autoantibody isotype and IgG subclasses were determined using the same approach.
Plasma Inhibition of IFNγ-Induced of pSTAT1 and Cytokine Production
Normal and patient PBMC were isolated by density gradient centrifugation as described [7] and cultured in complete RPMI 1640 media consisting of 2 mM glutamine, 20 mM Hepes, 100 U/mL penicillin, 100 μg/mL streptomycin with 10 % patient plasma, normal plasma, IgG fraction, or IgG-depleted flow-through until testing. Cultures were unstimulated or stimulated with IFNγ (1,000 U/mL; Intermune) or IFN-α2b (1,000 U/mL; Schering) for 15 min at 37 °C. Monocytes were identified by CD14 (BD Pharmingen) before being fixed and permeabilized for intranuclear staining using anti-phosphoSTAT-1 (Y701) antibody (BD Biosciences) as previously described [2]. Data were collected using FACS Calibur (BD Biosciences) and analyzed using FlowJo (Treestar).
Normal and patient PBMCs were incubated in complete RPMI media as above with either 10 % normal or patient plasma and left unstimulated or stimulated with PHA (1 %, Invitrogen) plus IL-12 (1 ng/mL; R&D) or LPS (200 ng/mL; Sigma-Aldrich) plus IFNγ (1,000 U/mL) for 48 h at 37 °C, 5 % CO2. Supernatants were tested for TNFα, IL-12p70, and IFNγ protein using the Bio-plex cytokine determination kit per manufacturer’s instructions (Bio-Rad).
Results
Our patient had a normal CD4 count of 1,144/uL (359–1,565/uL), a normal number of monocytes at 0.34 K/ul (0.24–0.86 K/uL), and normal INFγR1 expression. Her plasma had high-titer anti-IFNγ autoantibodies (Fig. 2a) exclusively of IgG isotype (not shown) and primarily of IgG1 subclass (Fig. 2b).
Fig. 2.
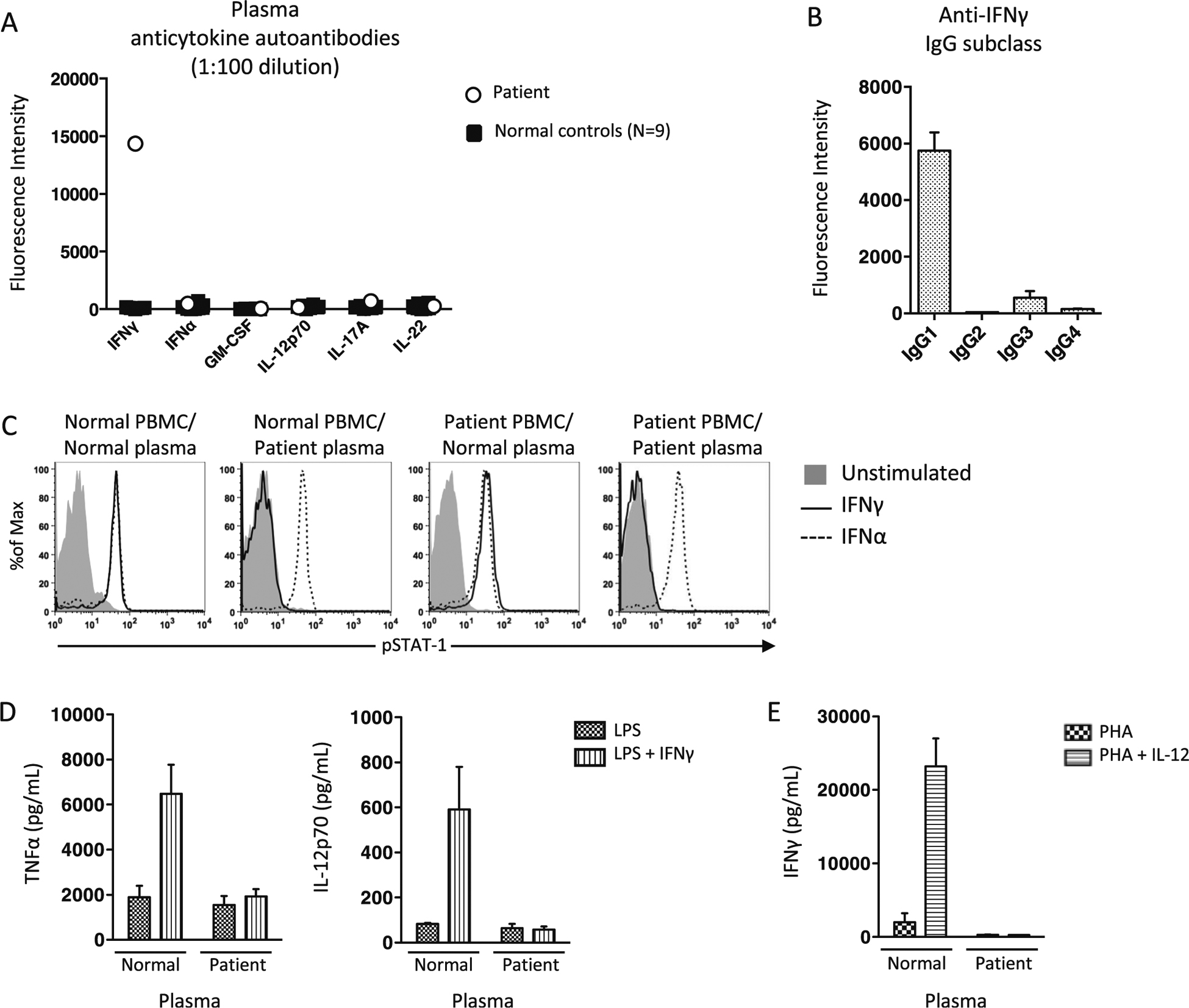
Identification and functional evaluation of anti-IFNγ autoantibodies. a Multiplex screen for anticytokine autoantibodies in our patient and normal control plasma (N=9). b Determination of IgG subclasses in patient plasma. c From left to right, normal PBMC incubated with normal and patient plasma or patient PBMC incubated with normal or patient plasma were left unstimulated or stimulated with IFNa or IFNg and evaluated for STAT-1 phosphorylation (pSTAT1). d Normal PBMC in the presence of normal and patient plasma were stimulated and supernatants collected for evaluation of IFNg-augmentation of LPS-induced TNFa and IL-12p70 and E. IL-12-augmentation of PHA-induced IFNγ production
Patient plasma, but not control plasma, inhibited IFNγ-induced pSTAT-1 formation in normal PBMC, whereas IFNα-induced pSTAT-1 formation was normal regardless of the plasma source (Fig. 2c). The IFNγ-blocking activity of patient plasma was limited to the patient’s IgG fraction (not shown). Patient PBMCs washed free of autologous plasma demonstrated both IFNγ and IFNα-induced pSTAT-1 formation in the presence of normal plasma (Fig. 2c). PBMCs incubated with patient but not normal plasma inhibited IFNγ augmentation of LPS-induced TNFα and IL-12p70 (Fig. 2d). Finally, patient but not normal plasma neutralized detection of PHA+IL-12-induced IFNγ (Fig. 2e).
Discussion
Unlike primary genetic immunodeficiencies which tend to present in childhood, anticytokine autoantibodies represent an emerging mechanism of infection susceptibility that are being increasingly recognized in previously healthy adults who develop severe opportunistic infections [8]. Like most of the more than 130 reported cases of immunodeficiency caused by anti-IFNγ antibodies, our patient was a previously healthy adult with extrapulmonary mycobacterial disease. However, unlike the majority of cases, our patient was neither of Asian origin or descent. To our knowledge, only 3 prior cases were not both Asian and Asian-born. They were three Caucasian residents of the UK, one of whom originated from South Africa (personal communication, Kampmann) [9]. The patient presented here is the first Caucasian born and raised on American soil with no known Asian ancestry suggesting this disease may extend beyond the currently appreciated ethnic and geographic boundaries. Interestingly, in the case of pulmonary alveolar proteinosis, a severe lung disease due to anti-GM-CSF autoantibodies, the clinical syndrome was recognized decades before anti-GM-CSF autoantibodies were identified as etiologic. Furthermore, it is now being recognized that anti-GM-CSF autoantibodies may predispose to other infections as well, independent of lung disease [10] and unpublished data), implicating anticytokine autoantibodies as an under-recognized mechanism of disease pathogenesis.
Anti-IFNγ autoantibodies have also been found in HIV [11, 12], other viral infections [13] African trypanosomiasis [14], pulmonary tuberculosis [15], and healthy controls [1], although their neutralizing capacity and biological significance remain unclear. Other work evaluating endogenous anti-TNFα antibodies in the context of rheumatoid arthritis (RA) suggests that anticytokine autoantibodies may be a physiologic strategy to mitigate an overexuberant inflammatory response [16]. The mechanism by which pathologic anti-IFNγ autoantibodies are produced has yet to be elucidated, however, as with other autoimmune disease, the confluence of strong ethnic, HLA [17], and geographic associations suggests a complex interaction of both host an environmental factors.
In this patient, IFNγ autoantibodies may account for the unusual extent and locations of her MAC disease. Reports of laryngeal nontuberculous mycobacterial infections have been only described in the context of inhaled steroid use [18, 19]. However, this patient was not on inhaled steroids at the time of development of subglottic MAC. Of the 6 previous reports of intracranial MAC in patients without HIV, 2 had extensive immunologic workups [20, 21]. One showed dysfunction in IFNγ signaling [20], while the other had low IFNγ and TNFα production compared to controls [21] suggesting a cell-intrinsic defect, and not autoantibodies.
Systemic and prolonged inhaled steroids in asthmatics have been associated with the development of pulmonary MAC [22, 23], as well as delayed sputum conversion [24], but they have not been associated with disseminated NTM disease. Further, steroids were administered contemporaneously in response to her initial presentation for tracheal disease, decreasing the likelihood of a causal relationship, although this cannot be excluded completely as a contributing factor thereafter. We believe that steroids alone could not explain the recurrent and multifocal nature of her disseminated MAC infection and that her IFNγ autoantibodies played a significant role in her disease.
Rituximab has been used in some patients to reduce titers of anti-IFNγ antibodies, thereby enabling clearance of mycobacterial infection [25], but does not appear to be necessary in all cases. Stopping oral corticosteroids and improved adherence to anti-infectives alone led to disease resolution. She currently remains on azithromycin, ethambutol, rifampin and levofloxacin and has no evidence of active infection.
Conclusions
Disseminated NTM infection in non-HIV patients indicates significant immunologic dysfunction and should prompt immunologic evaluation, in particular investigation of the IFNγ-IL12 pathway. Anti-IFNγ autoantibodies can impair cytokine signaling, and do not appear to be solely limited to people of Southeast Asian origin or descent.
Acknowledgments
Supported by the Division of Intramural Research, National Institute of Allergy and Infectious Diseases, National Institutes of Health. Johns Hopkins Hospital.
References
- 1.Browne SK, Burbelo PD, Chetchotisakd P, Suputtamongkol Y, Kiertiburanakul S, Shaw PA, et al. Adult-onset immunodeficiency in Thailand and Taiwan. N Engl J Med 2012;367(8):725–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Patel SY, Ding L, Brown MR, Lantz L, Gay T, Cohen S, et al. Anti-IFN-gamma autoantibodies in disseminated nontuberculous mycobacterial infections. J Immunol. 2005;175(7):4769–76. [DOI] [PubMed] [Google Scholar]
- 3.Koya T, Tsubata C, Kagamu H, Koyama K, Hayashi M, Kuwabara K, et al. Anti-interferon-gamma autoantibody in a patient with disseminated mycobacterium avium complex. J Infect Chemother Off J Jpn Soc Chemother 2009;15(2):118–22. [DOI] [PubMed] [Google Scholar]
- 4.Doffinger R, Helbert MR, Barcenas-Morales G, Yang K, Dupuis S, Ceron-Gutierrez L, et al. Autoantibodies to interferon-gamma in a patient with selective susceptibility to mycobacterial infection and organ-specific autoimmunity. Clin Infect Dis Off Publ Infect Dis Soc Am 2004;38(1):e10–4. [DOI] [PubMed] [Google Scholar]
- 5.C-CC Chih-Yu Chi, Jing-Pei L, Chia-Hao L, Mao-Wang H, Wen-Jyi L, Po-Chang L, et al. Anti-IFN-γ autoantibodies in adults with disseminated nontuberculous mycobacterial infections are associated with HLA-DRB1*16:02 and HLA-DQB1*05:02 and the reactivation of latent varicella-zoster virus infection. Blood 2013;121(8):1357–66. [DOI] [PubMed] [Google Scholar]
- 6.Ding Q, Yeung M, Camirand G, Zeng Q, Akiba H, Yagita H, et al. Regulatory B cells are identified by expression of TIM-1 and can be induced through TIM-1 ligation to promote tolerance in mice. J Clin Invest 2011;121(9):3645–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Holland SM, Dorman SE, Kwon A, Pitha-Rowe IF, Frucht DM, Gerstberger SM, et al. Abnormal regulation of interferon-gamma, interleukin-12, and tumor necrosis factor-alpha in human interferon-gamma receptor 1 deficiency. J Infect Dis 1998;178(4):1095–104. [DOI] [PubMed] [Google Scholar]
- 8.Browne SK. Anticytokine autoantibody-associated immunodeficiency. Annu Rev Immunol 2014;32:635–57. [DOI] [PubMed] [Google Scholar]
- 9.Kampmann B, Hemingway C, Stephens A, Davidson R, Goodsall A, Anderson S, et al. Acquired predisposition to mycobacterial disease due to autoantibodies to IFN-gamma. J Clin Invest 2005;115(9): 2480–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rosen LB, Freeman AF, Yang LM, Jutivorakool K, Olivier KN, Angkasekwinai N, et al. Anti-GM-CSF autoantibodies in patients with cryptococcal meningitis. J Immunol (Baltimore Md 1950) 2013;190(8):3959–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.De Francesco MA, Caruso A, Dima F, Cantalamessa A, Canaris AD, Folghera S, et al. IFN-gamma restores HIV- and non-HIV-specific cell mediated immune response in vitro and its activity is neutralized by antibodies from patients with AIDS. Scand J Immunol 1996;43(1):94–100. [DOI] [PubMed] [Google Scholar]
- 12.Caruso A, Foresti I, Gribaudo G, Bonfanti C, Pollara P, Dolei A, et al. Anti-interferon-gamma antibodies in sera from HIV infected patients. J Biol Regul Homeost Agents 1989;3(1):8–12. [PubMed] [Google Scholar]
- 13.Caruso A, Bonfanti C, Colombrita D, De Francesco M, De Rango C, Foresti I, et al. Natural antibodies to IFN-gamma in man and their increase during viral infection. J Immunol (Baltimore Md 1950) 1990;144(2):685–90. [PubMed] [Google Scholar]
- 14.Bonfanti C, Caruso A, Bakhiet M, Olsson T, Turano A, Kristensson K. Increased levels of antibodies to IFN-gamma in human and experimental African trypanosomiasis. Scand J Immunol 1995;41(1):49–52. [DOI] [PubMed] [Google Scholar]
- 15.Madariaga L, Amurrio C, Martin G, Garcia-Cebrian F, Bicandi J, Lardelli P, et al. Detection of anti-interferon-gamma autoantibodies in subjects infected by mycobacterium tuberculosis. Int J Tuberc Lung Dis Off J Int Union against Tuberc Lung Dis 1998;2(1):62–8. [PubMed] [Google Scholar]
- 16.Wildbaum G, Nahir MA, Karin N. Beneficial autoimmunity to pro-inflammatory mediators restrains the consequences of self-destructive immunity. Immunity 2003;19(5):679–88. [DOI] [PubMed] [Google Scholar]
- 17.Ku CJ, Hosoya T, Maillard I, Engel JD. GATA-3 regulates hematopoietic stem cell maintenance and cell-cycle entry. Blood. 2012;119(10) :2242–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McEwan JA, Mohsen AH, Schmid ML, McKendrick MW. A hoarse voice: atypical mycobacterial infection of the larynx. J Laryngol Otol. 2001;115(11):920–2. [DOI] [PubMed] [Google Scholar]
- 19.Wang BY, Amolat MJ, Woo P, Brandwein-Gensler M. Atypical mycobacteriosis of the larynx: an unusual clinical presentation secondary to steroids inhalation. Ann Diagn Pathol 2008;12(6):426–9. [DOI] [PubMed] [Google Scholar]
- 20.Sadek M, Yue FY, Lee EY, Gyenes G, Jones RB, Hoffstein V, et al. Clinical and immunologic features of an atypical intracranial mycobacterium avium complex (MAC) infection compared with those of pulmonary MAC infections. Clin Vaccine Immunol CVI 2008;15(10):1580–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dickerman RD, Stevens QE, Rak R, Dorman SE, Holland SM, Nguyen TT. Isolated intracranial infection with mycobacterium avium complex. JNeurosurg Sci 2003;47(2):101–5. discussion 5. [PubMed] [Google Scholar]
- 22.Fritscher LG, Marras TK, Bradi AC, Fritscher CC, Balter MS, Chapman KR. Nontuberculous mycobacterial infection as a cause of difficult-to-control asthma: a case-control study. Chest 2011. ;139(1):23–7. [DOI] [PubMed] [Google Scholar]
- 23.Dirac MA, Horan KL, Doody DR, Meschke JS, Park DR, Jackson LA, et al. Environment or host?: a case-control study of risk factors for mycobacterium avium complex lung disease. Am J Respir Crit Care Med 2012;186(7):684–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kobashi Y, Matsushima T. Clinical analysis of pulmonary mycobacterium avium complex disease in association with corticosteroid treatment. J Infect Chemother Off J Jpn Soc Chemother 2003;9(1): 68–74. [DOI] [PubMed] [Google Scholar]
- 25.Browne SK, Zaman R, Sampaio EP, Jutivorakool K, Rosen LB, Ding L, et al. Anti-CD20 (rituximab) therapy for anti-IFN-gamma autoantibody-associated nontuberculous mycobacterial infection. Blood 2012;119(17):3933–9. [DOI] [PMC free article] [PubMed] [Google Scholar]


