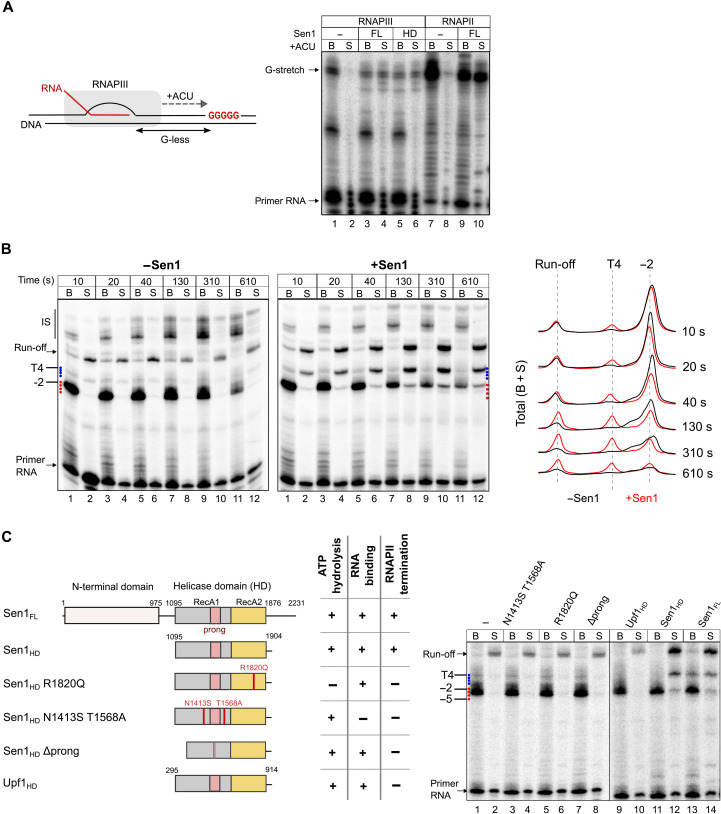
Fig. 6. Analysis of the mechanisms of Sen1-mediated termination of RNAPIII transcription.
(A) Analysis of the capacity of Sen1 to promote the release of RNAPIII paused at a sequence other than a T-tract. The transcription templates contain a G-less cassette, followed by a stretch of Gs to induce RNAPIII stalling at the first G in the absence of GTP. Experiments with purified RNAPII were performed as a positive control for Sen1 activity and provided results similar to our former studies (19, 21). (B) Time-course in vitro transcription termination assay performed on transcription templates containing a T4 terminator. All reactions were performed in parallel but migrated on different gels. Left: Representative gel of one of two independent experiments. Right: Profile of the total signal (beads + supernatant) over the region of interest. (C) Analysis of the role of different protein regions and activities of Sen1 in RNAPIII transcription termination. Left: Scheme of the different proteins used in in vitro transcription termination assays and summary of the relevant phenotypes. The different variants of Sen1 helicase domain (HD) were purified and characterized within the frame of a previous study (21). A constitutively active version of the Upf1 helicase domain that has helicase activity but cannot induce RNAPII transcription termination in vitro is used as a negative control [see (14)]. The Δprong version of Sen1 helicase domain contains a deletion of amino acids 1461 to 1554 corresponding to most of this subdomain. Right: Representative gel of one of two independent experiments. All reactions were performed in parallel but migrated on different gels. IS, iterative synthesis.