Extended Data Fig. 3 |. The tumor suppressor FLCN specifically binds to the amino and carboxy domain of LDHA.
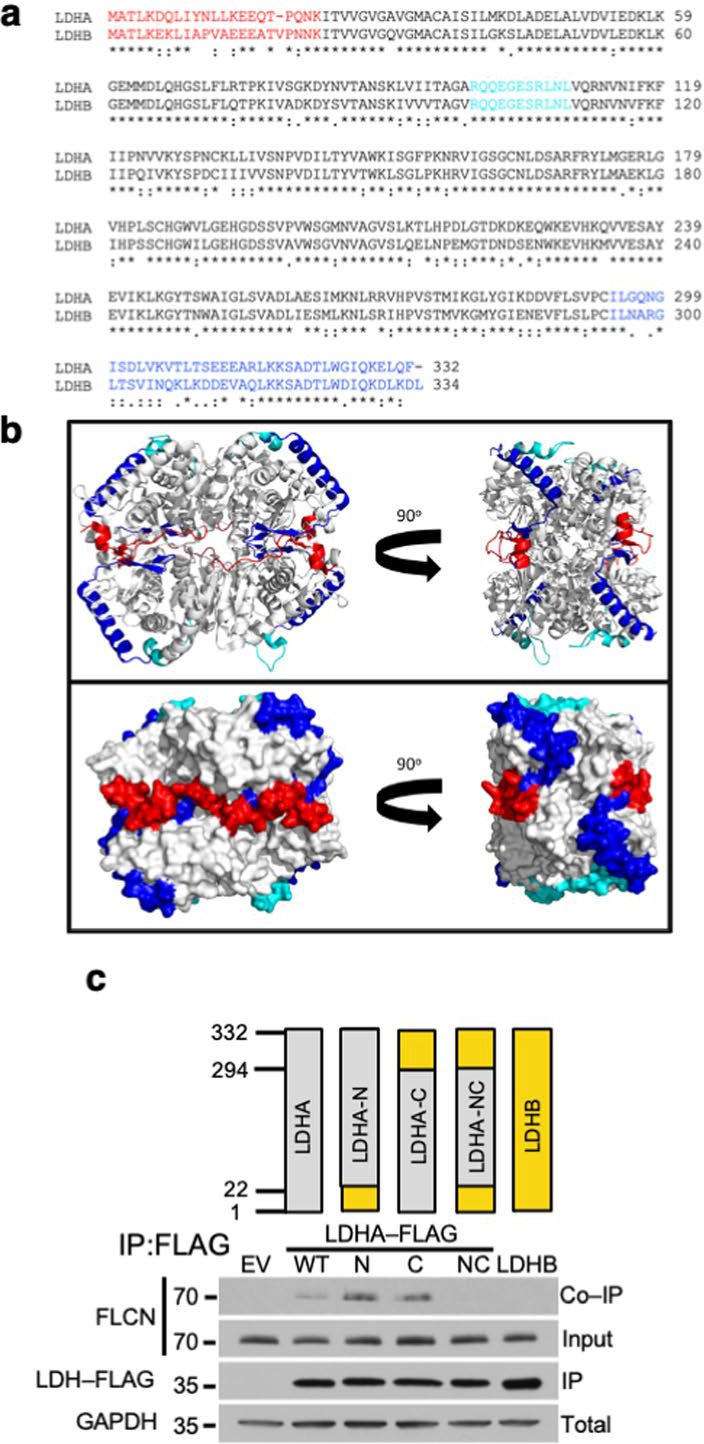
a) Sequence alignment of human LDHA and human LDHB using https://clustalw.ddbj.nig.ac.jp/. The red N- and blue C-terminal residues that were swapped to generate the N, C and NC chimeric constructs used in Extended Data Fig. 2c are highlighted. The active site loop (99–110) is colored cyan. b) LDHA tetramer (PDB ID: 1i10) in ribbon (top) and surface (bottom) models represented in the standard view (left) and 90° rotated view (right). The N-terminus (residues 1–22) is highlighted in red and the C-terminus (residues 294–332) is highlighted in blue. Note the proximity of the C-terminal helix (blue) to the active site loop (residues 99–110; cyan) within each subunit. In the tetrameric arrangement, the N- and C-terminal residues of adjacent subunits form a continuous surface. Structure rendered using PyMOL 2.3. c) LDHA/LDHB chimeric constructs were transfected into HEK293 cells and immunoprecipitated. Co-IP of FLCN was detected by immunoblot.
