Abstract
Diabetes mellitus (DM) is a typical chronic disease that can be divided into 2 types, dependent on insulin deficiency or insulin resistance. Incidences of diabetic complications gradually increase as the disease progresses. Studies in diabetes complications have mostly focused on kidney and cardiovascular diseases, as well as neuropathy. However, DM can also cause skeletal muscle atrophy. Diabetic muscular atrophy is an unrecognized diabetic complication that can lead to quadriplegia in severe cases, seriously impacting patients’ quality of life. In this review, we first identify the main molecular mechanisms of muscle atrophy from the aspects of protein degradation and synthesis signaling pathways. Then, we discuss the molecular regulatory mechanisms of diabetic muscular atrophy, and outline potential drugs and treatments in terms of insulin resistance, insulin deficiency, inflammation, oxidative stress, glucocorticoids, and other factors. It is worth noting that inflammation and oxidative stress are closely related to insulin resistance and insulin deficiency in diabetic muscular atrophy. Regulating inflammation and oxidative stress may represent another very important way to treat diabetic muscular atrophy, in addition to controlling insulin signaling. Understanding the molecular regulatory mechanism of diabetic muscular atrophy could help to reveal new treatment strategies.
Keywords: diabetes mellitus, muscle atrophy, molecular mechanism, treatment, inflammation
Introduction
Diabetes mellitus (DM) is a common chronic metabolic disease. There are two main subtypes of endocrine cells in pancreatic islets: β cells and α cells. Islet β cells are involved in the production of insulin, while islet α cells are responsible for the secretion of glucagon. These cell types work together to maintain an appropriate blood glucose level in the human body. DM can be mainly divided into type 1 diabetes mellitus (T1DM) and type 2 diabetes mellitus (T2DM). T1DM accounts for less than 10% of all instances of DM, with T2DM accounting for more than 90% (1). DM is mainly induced by two causes: the impairment of insulin secretion (insulin deficiency) and insulin resistance (2). The former results from islet β cell dysfunction, while the latter refers to the loss of insulin-mediated cellular glucose uptake in DM patients. Increased insulin levels reduce the affinity of insulin receptors, meaning that cells gradually become insensitive to insulin.
DM is often accompanied by secondary complications that involve multiple organs, such as the eyes, kidneys, heart, and brain, as well as skeletal muscle (3). To date, relevant studies have mainly discussed the risks of cardiovascular disease, blindness, and renal failure in DM patients (4). In addition, DM also induces a shift in muscle fiber phenotype from slow-twitch to fast-twitch, which can lead to skeletal muscle atrophy, energy metabolism disorders, and muscle weakness (1, 4). Muscle atrophy is caused by an imbalance between the synthesis and degradation of protein (5). Maintaining muscle homeostasis is crucial for preserving the body’s integrity and function. Muscle atrophy has also been associated with a variety of diseases, and can lead to a poor quality of life. Diabetic muscular atrophy is considered to be a DM complication; it is characterized by proximal lower extremity muscle weakness, atrophy, pain, sensory disturbances, and even quadriplegia in severe cases (6). Research into the molecular mechanism of diabetic muscular atrophy and its treatment strategies could aid the development of effective treatments and improve prognoses. To date, however, little research has been conducted in this regard.
Muscle atrophy is closely related to two major protein degradation pathways, the ubiquitin-proteasome system (UPS) and the autophagy-lysosome pathway (ALP). It is also related to the protein synthesis pathways, such as the insulin-like growth factor 1– phosphoinositide-3-kinase–Akt/protein kinase B–mammalian target of rapamycin (IGF1–PI3K–Akt/PKB–mTOR) pathway and IGF-1-AKT- Forkhead box O (FoxO) pathways (7–12). In T2DM, insulin resistance has been shown to inhibit protein synthesis by inhibiting the IGF-1-PI3K-AKT/PKB-mTOR pathway, and to activate the UPS and ALP through the IGF-1-AKT-FoxO signaling pathway, thereby promoting muscle atrophy. T1DM-induced muscle atrophy, meanwhile, is mediated by the FoxO-driven protein degradation pathway (13–15). In addition, oxidative stress damage, inflammatory response, and high levels of glucocorticoids (GCs) can all trigger muscle atrophy in DM patients (16, 17). Herein, we discuss the major molecular regulatory mechanisms of muscle atrophy and diabetic muscular atrophy, and describe potential drugs and treatments for the latter. Moreover, we provide new ideas and strategies to improve the prognosis of DM patients.
Molecular Mechanisms of Skeletal Muscle Atrophy
Protein Degradation Pathways
The UPS is the major hydrolysis system for cellular proteins; it is responsible for the degradation of misfolded or damaged cellular proteins in skeletal muscle (18, 19). Ubiquitin is a short protein comprising 76 amino acids; it can activate proteolysis in skeletal muscle (20). Most proteins are degraded by the 26S proteasome, through the modification of covalently attached polyubiquitin chains. The ubiquitination of proteins proceeds through a cascade of reactions that are catalyzed by a range of enzymes, including E1 ubiquitin-activating enzyme, E2 ubiquitin-conjugating enzyme, and E3 ubiquitin ligase (21). These tagged proteins are subsequently recognized by the 26S proteasome, which consists of a 20S core and two 19S regulatory complexes. These 19S regulatory complexes can recognize and bind to ubiquitinated proteins to undergo specific proteolysis (22). Cullin-RING E3 ubiquitin ligases (CRL) are the largest known class of ubiquitin ligases; they regulate a variety of cellular processes in skeletal muscle, including cellular proliferation, transcription, signal transduction, and development (23). Levels of the muscle-specific E3 ubiquitin ligases muscle RING-finger protein-1 (MuRF1) and muscle atrophy F-box (MAFbx)/Atrogin-1 have been shown to be significantly upregulated in atrophic skeletal muscle. These ligases are also known to be involved in the degradation of skeletal muscle proteins (24–26). Therefore, the UPS is one of the main mechanisms underlying muscle atrophy, and MuRF1 and MAFbx are two important regulators of muscle atrophy.
The ALP is a lysosomal degradation pathway that is widespread in eukaryotic cells; it is involved in the degradation and elimination of damaged, degenerated, aged, or dysfunctional organelles (27). The ALP is crucial for cell survival and repair, intracellular protein balance, and environmental homeostasis. Under starvation and other conditions, a double-layered membrane structure forms in cells; this structure then gradually extends to envelop undegraded proteins or cellular components, thereby forming autophagosomes. Autophagosomes are driven by cytoskeletal proteins to fuse with lysosomes, where their cargo components become degraded (28). Autophagy has dual functions. On the one hand, it can degrade damaged organelles and abnormal proteins, preventing them from accumulating in cells. On the other hand, autophagy overactivation can damage organelles, and thereby become toxic to cells. Studies have found that autophagy is crucial for maintaining skeletal muscle homeostasis, and that overactivated autophagy can promote muscle atrophy or autophagy impairment, leading to muscle degeneration (29, 30). Autophagy is activated under nutrient deprivation. Antimicrobial peptide-activated protein kinase (AMPK) inhibits the mTOR complex-1 (mTORC1) complex, which in turn inhibits protein synthesis, and can even lead to severe late-onset myopathy (31, 32). The studies demonstrate that autophagy plays an important role in skeletal muscle atrophy.
Protein Synthesis Pathway
The IGF-1-PI3K-AKT-mTOR pathway is a positive regulator that is responsible for controlling protein synthesis (33). IGF-1 is a key growth factor that regulates skeletal muscle synthesis and catabolism; it also promotes the growth of muscle cells. The inactivation of muscle-specific IGF-1 receptors impairs muscle growth, triggering a reduction in the number, diameter, and cross-sectional area of muscle fibers (34). Conversely, the overexpression of muscle-specific IGF-1 receptors has been shown to lead to muscle hypertrophy (11). Then, IGF-1 can activate the PI3K-AKT signaling pathway and then stimulate mTOR activity (35). mTOR is assembled into different complexes such as mTORC1 and mTOR complex-2 (mTORC2). mTORC1 positively regulates the activation of its effector 70-kDa ribosomal protein S6 kinase (p70S6K) and negatively regulates the inhibitory of the translation initiation factor 4E-eukaryotic translation initiation factor 4E binding protein 1 (eIF4E-4EBP1) complex. This leads to increased protein translation and synthesis, which subsequently promotes muscle growth (36, 37).
In addition to IGF-1’s regulatory role in muscle growth and protein synthesis, the FoxO family also plays an important role in the pathophysiological process of skeletal muscle development. The FoxO family has three subtypes, FoxO1, FoxO3, and FoxO4, which control a series of atrophy-related genes in skeletal muscle, including MAFbx and MuRF1. The dephosphorylation of FoxO members can up-regulate MAFbx and MuRF1, thereby accelerating protein degradation and subsequently inducing muscle atrophy (5). FoxO’s transcription factor is the downstream target of the PKB/AKT pathway. AKT can phosphorylate all members of FoxO, with the resulting phosphorylated FoxO members being exported from the nucleus to the cytoplasm. This inhibits their transcriptional activity, and eventually represses muscle atrophy (38). Although AKT can inhibit the UPS and ALP (5), AKT remains inactive in the absence of growth factors. FoxO members will translocate to the nucleus and induce the transcription of the target genes of UPS and ALP (9), thereby initiating protein degradation.
Molecular Mechanism of Diabetic Muscular Atrophy
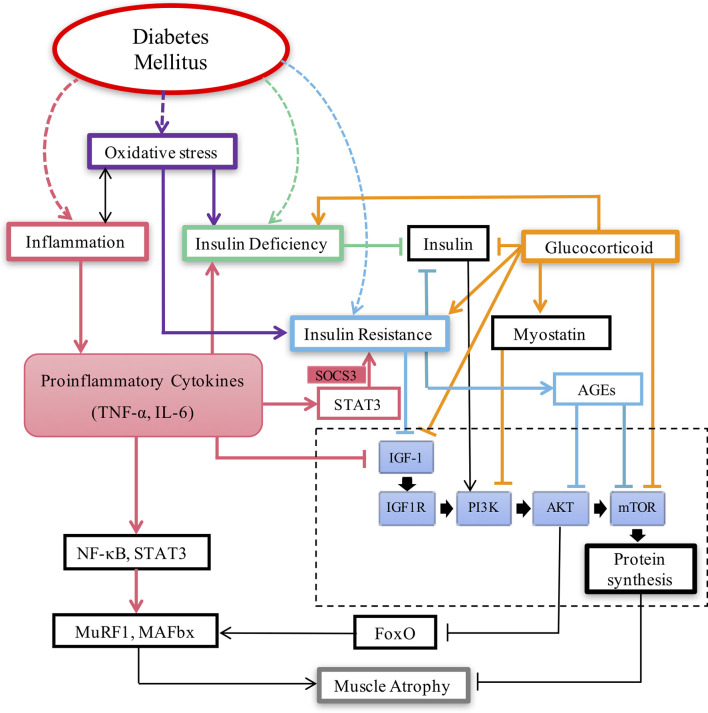
The molecular mechanism of diabetic muscular atrophy is very complicated. The current mainstream view is that diabetic muscular atrophy is closely related to insulin resistance, insulin deficiency, inflammation, oxidative stress, glucocorticoids and so on ( Figure 1 ).
Figure 1.
Key pathways involved in Diabetic muscular atrophy.
Insulin Resistance
Insulin-stimulated glucose uptake is crucial for muscle contraction. Insulin is a powerful synthetic signal that significantly stimulates muscle protein synthesis (39). Classical insulin signaling pathways and anabolic stimuli, including PI3K, 3-phosphoinositide-dependent kinase 1 (PDK1), AKT, mTOR, and p70S6K, activate protein synthesis and thereby promote muscle growth (40, 41). DM-induced insulin dysfunction inhibits glucose uptake in skeletal muscle, thereby disturbing muscle contraction (42). Under normal conditions, the intracellular insulin signaling cascade activates the mTOR pathway and inhibits autophagy (including the lysosomal degradation of proteins and organelles). However, such effects are deactivated in the presence of insulin resistance, which may accelerate muscle loss in patients with DM (43). In addition, sarcopenia is a complication of T2DM that is characterized by the progressive loss of skeletal muscle mass and function (44, 45). Sarcopenia is related to the weakness and geriatric syndrome of the human body; it can impact quality of life for elderly patients, and can even lead to death in severe cases (46). Due to low muscle mass, sarcopenia may lead to insulin resistance through altered glucose disposal (47).
When insulin resistance occurs, insulin or IGF-1 signaling is inhibited. This results in the inactivation of the PI3K/AKT pathway, which in turn inhibits mTOR activity and reduces protein synthesis; these effects may ultimately lead to muscle loss in T2DM patients (48). In addition, insulin resistance leads to elevated systemic glucose levels, with glucose being able to react with proteins or lipids to generate advanced glycation end products (AGEs) (49). AGEs play an important role in the pathogenesis of chronic diabetic complications. Moreover, the accumulation of AGEs is a potential cause of muscle loss and muscle weakness in T2DM patients (50). Receptor for advanced glycation end products (RAGE) is a transmembrane signaling receptor that is associated with diabetic renal and vascular complications. AGEs can induce muscle atrophy or myogenesis impairment through the RAGE-mediated, AMPK-induced downregulation of AKT signaling (51). Furthermore, AGEs have been shown to modulate muscle anabolic signaling by inhibiting the mTORC1 signaling pathway (50). Overall, insulin resistance can inactivate the IGF-1-PI3K-AKT-mTOR protein synthesis pathway, thereby reducing protein synthesis and ultimately inducing skeletal muscle atrophy.
Insulin Deficiency
Patients with T1DM exhibit a reduced repair capacity regarding their skeletal muscle satellite cells, as well as skeletal muscle dysfunction. Both of abnormal phenotypes are associated with insulin deficiency, which causes the rates of protein degradation exceed that of protein synthesis (52). Under normal conditions, insulin receptor (IR) and IGF-1 receptor (IGF-1R) can act through the PI3K/AKT pathway on a variety of cellular functions. For example, during glucose uptake and protein synthesis, the activation of AKT in response to insulin or IGF-1 can phosphorylate FoxO transcription factors, thereby inhibiting their transcriptional activity (4). Insulin-deficient diabetes, or a loss of insulin/IGF-1 action in muscle, reduces complex I-driven mitochondrial respiration and supercomplex assembly through the FoxO-mediated inhibition of complex I subunit (53). These effects impact mitochondrial function and induce skeletal muscle atrophy. In a biopsy from patients with T1DM who experienced insulin deficiency for 8 h, transcripts of the UPS and ALP were found to be increased, indicating that muscle atrophy in T1DM is induced by FoxO-driven protein degradation. Therefore, blocking this pathway may protect against diabetic complications (4). In short, when insulin is deficient, the transcriptional activity of FoxO becomes enhanced, which in turn promotes the expression of muscle atrophy-related genes (such as MAFbx and MuRF1) and causes muscle atrophy.
Inflammation
Interleukin-6 (IL-6) is a pro-inflammatory cytokine that has catabolic effects on muscle (54). Patients with T2DM have elevated levels of C-reactive protein, IL-1β, and IL-6 (55). In T1DM, the ability of skeletal muscle regeneration is impaired due to dysfunction of satellite cells. The transient elevation of IL-6 leads to the proliferation of satellite cells, while the slow elevation of IL-6 impairs satellite cell function (56). Therefore, chronically elevated IL-6 levels may be responsible for satellite cell dysfunction in DM. In addition, hyperglycemia can promote the release of inflammatory mediators, such as IL-6. It can also stimulate macrophages, some other innate immune cells, and activate some apoptosis-related signaling pathways, such as the Fas/FasL signaling pathway (57, 58). Such stimulation can lead to islet β cell dysfunction, subsequently causing insulin deficiency (2). In addition, IL-6 can also induce insulin resistance by reducing insulin sensitivity, or by affecting lipid metabolism (59, 60). Insulin acts by binding to IR. Tumor necrosis factor alpha (TNF-α), which is a pro-inflammatory cytokine, can destroy the tyrosine phosphorylation activation of IR and IR substrate (IRS) in the insulin signaling cascade, thereby leading to insulin resistance (60). In addition, TNF-α can also reduce the glucose uptake and utilization of skeletal muscle and adipocytes by reducing the expression of glucose transporter 4 (GLUT4). This leads to insulin resistance (61), thereby promoting muscle atrophy.
Furthermore, the systemic inflammatory responses caused by obesity and long-term overnutrition not only induce typical insulin resistance in T2DM patients, but also reduce protein synthesis in muscle, thereby promoting UPS- and ALP-mediated protein degradation and facilitating the progression of muscle atrophy (62). Signal transducer and activator of transcription 3 (STAT3) can be activated by pro-inflammatory cytokines (such as IL-6); this weakens protein synthesis-related signaling pathways in muscle (63–66). Nuclear factor-κB (NF-κB) is an important transcriptional regulator that can induce the expression of various genes by activating stimulatory factors (viruses, tumor necrosis factor, and B cell activating factor) (67, 68). Additionally, NF-κB can increase the degradation of specific muscle proteins by increasing the expression of MuRF1 (69–71). NF-κB and STAT3 signaling pathways function as inflammatory pathways that can be significantly activated by increases in pro-inflammatory cytokines (i.e., TNF-α) and non-esterified fatty acids. This then increases the expression of MuRF1, thereby activating the UPS (72, 73). IL-6-activated STAT3 can induce insulin resistance through suppressors of cytokine signaling 3 (SOCS3); it can then inhibit the PI3K-AKT pathway, which reduces protein synthesis (74, 75) and increases myostatin transcription (72). These effects are significant as they can improve the progression of muscle atrophy. NF-κB and STAT3 may be involved in muscle atrophy in T2DM patients (62). In addition, IL-6 can also induce muscle atrophy via the regulation of IGF-1 (37, 74). Overall, inflammation can reduce protein synthesis through the inhibition of IGF-1 and the induction of insulin resistance, activate the UPS through the FoxO family and their downstream E3 ubiquitin ligases, and promote the expression of atrophy-related genes (possibly through the NF-κB and STAT3 pathways). Thus, it can eventually lead to muscle atrophy.
Oxidative Stress
Excess production of reactive oxygen species (ROS) in the human body can induce oxidative stress, which damages lipids, proteins, and deoxyribonucleic acid (DNA) (76). In addition, obesity and hyperglycemia can also lead to oxidative stress (77–79). The high metabolic capacity of skeletal muscle makes it susceptible to oxidative stress injuries (37). Oxidative stress inhibits the AKT-mTOR pathway and its downstream targets, which subsequently inhibits protein synthesis and promotes muscle atrophy (80, 81). In addition, islet β cells are particularly sensitive to ROS due to their inherent antioxidant enzymes at low levels. ROS can directly damage β cells and promote apoptosis. Moreover, they can indirectly regulate the insulin signaling pathway and inhibit the function of β cells, leading to the occurrence of DM (82, 83). ROS is an important mediator for activating pro-inflammatory signaling pathways (84, 85). A chronic inflammatory environment is also conducive to producing free radicals, such as ROS. This can aggravate β-cell damage, thereby generating a positive feedback loop in which further harmful cytokines are then secreted, triggering further damage to β cells (86). Oxidative stress can induce insulin deficiency, and can produce large quantities of ROS to hinder insulin signaling transduction, thereby triggering insulin resistance (87). Eventually, this can contribute to the development of skeletal muscle atrophy. Therefore, oxidative stress injury plays an key role in the process of skeletal muscle atrophy.
GCs
GC is a hypoglycemic hormone that promotes gluconeogenesis and glycogen breakdown, thereby counteracting the action of insulin and increasing blood glucose levels (88). GC signaling significantly contributes to muscle atrophy in DM (89). In addition, when GC binds to glucocorticoid receptor (GR), it inhibits AKT, GLUT4, and IR signals, and then induces insulin resistance (90). In T1DM caused by insulin deficiency, when insulin deficiency coexists with GCs in muscle, GR can compete with IRS1 to bind PI3K subunits P110 and p85. This results in a decrease in the phosphorylation levels of IRS, PI3K, and AKT, eventually leading to muscle atrophy (89, 90). GCs mainly cause muscle atrophy by increasing protein breakdown through the UPS and ALP, and by reducing protein synthesis via the inhibition of the IGF-1-PI3K-AKT-mTOR and mTOR/p70S6k pathways (91–93). In addition, GCs upregulate the production of myostatin, which reduce protein synthesis via the AKT-mTOR pathway (94). GCs can also induce muscle atrophy by binding to their receptors, thereby interfering with the insulin/IGF-1 signaling pathway and stimulating the transcription of dystrophin. Moreover, GRs can synergize with FoxO1 to induce MuRF1, thereby accelerating muscle atrophy (95). GRs also upregulate the expression of regulated in development and DNA damage responses 1 (REDD1) and Kruppel like factor 15 (KLF15). KLF15 is a member of the KLF transcription factor family; it can regulate muscle catabolism by regulating MAFbx and MuRF1 (96). The expression of KLF15 has been found to be up-regulated in the livers of diabetic mice, and hyperglycemia is known to up-regulate KLF15 protein, thereby accelerating skeletal muscle atrophy (97). REDD1 is a stress-responsive protein that inhibits the targets of mTOR in mTOR1 (98). The inhibition of mTOR can upregulate KLF15, which would increase the expression of atrophy-related genes, thereby triggering atrophy (96, 99). Overall, GCs participate in skeletal muscle atrophy through multiple pathways.
Other Factors
Glucose can stimulate the degradation of WW domain-containing E3 ubiquitin protein ligase 1 (WWP1) through the proteasome pathway. The downregulation of WWP1 inhibits the ubiquitin-dependent degradation of KLF15 and then up-regulates KLF15 expression. This increases the expression of muscle atrophy-related genes, resulting in the loss of skeletal muscle mass (100). IGF-1 activates PDK1 to exert a core role in anabolic signaling. Subsequently, PDK1 activates the AKT/mTOR/p70S6k pathway (101), which further promotes protein synthesis. Moreover, it inhibits protein degradation by inhibiting the FoxO1 transcription factor (102). Increasing amounts of evidence have shown that microRNAs are involved in the regulation of skeletal muscle homeostasis. The overexpression of miR-193b in T1DM patients has been shown to reduce the expression of PDK1 and the phosphorylation of AKT, mTOR, p70S6k, and AMPK. In this way, it can inhibit protein synthesis and enhance the expression of MAFbx and MuRF1, thereby promoting proteolysis (103). To summarize, the mechanism that causes diabetic muscular atrophy is very complex; it warrants further in-depth study and exploration.
Treatments and Therapeutic Drugs
To gain a systematic understanding of the impact of commonly used anti-diabetic drugs on muscle atrophy and discuss potential therapeutic drugs, we outline various studies on the drugs to treat diabetic muscular atrophy ( Table 1 ).
Table 1 .
Treatments and therapeutic drugs for diabetic muscular atrophy.
| Drugs | Function | Mechanism | Effect on muscle | References |
|---|---|---|---|---|
| Metformin | Targeted insulin resistance | Activate AMPK | Promote repair of skeletal muscle | (104, 105) |
| Anti-inflammatory | Inhibit NF-κB | (106) | ||
| Thiazolidinedione | Targeted insulin resistance | Inhibition of protein hydrolysis and induction of PGC-1α to reduce the expression of atrophy-related genes | Attenuate the muscle wasting | (104) |
| Insulin | Targeted insulin deficiency | Promote the synthesis of protein; inhibit the decomposition of protein | Indirect benefit to skeletal muscle | (104) |
| Aspirin | Antioxidation | Reduce the production of ROS | Attenuate muscle wasting | (66) |
| Anti-inflammatory | Inhibition of Janus Kinase (JAK)/STAT and NF-κB signaling pathway | (107) | ||
| Targeted insulin resistance | ||||
| Omega-3 fatty acid | Antioxidation | Inhibit the production of ROS | Against Muscle atrophy | (108) |
| Anti-inflammatory | Decreased Activation of NF-κB; stimulated MTORC1 signal | (109–111) | ||
| Vitamin D | Antioxidation | Get rid of ROS | Prevent and cure muscle atrophy | (112) |
| Anti-inflammatory | The proinflammatory cascade reaction (NF-κB, TNF-α) was down-regulated, and the expression of FOXO1 was decreased | (112, 113) |
Targeting Insulin Resistance and Insulin Deficiency
The commonly used drugs for T2DM include metformin, glinides, thiazolidinediones, and peptidyl peptidase-4 inhibitors, all of which can improve insulin sensitivity. Metformin is a first-line drug for T2DM; it can eliminate the deleterious effects of DM on human bones. By activating AMPK, metformin can not only increase the translocation of glucose transporter 4 to the cell membrane, but can also contribute to skeletal muscle repair (104, 105). Glinides stimulate insulin secretion to reduce blood glucose level by closing the ATP-sensitive potassium channels (KATP) channel of islet β cells (114). However, repaglinide, which is a kind of glinide, can induce skeletal muscle atrophy and sarcopenia (104). Therefore, this drug may be not the ideal therapeutic drug of DM. Thiazolidinediones function as an important class of insulin sensitizers; they can not only improve insulin sensitivity to DM (115), but can also inhibit proteolytic pathways and stimulate mitochondrial biogenesis. Thiazolidinediones can partially induce the expression of peroxisome proliferator-activated receptor-gamma coactivator 1 alpha (PGC-1α), so as to reduce the expression of atrophy-related genes in muscles of patients with T2DM (104). Thus, they may be able to prevent diabetic muscular atrophy. Insulin remains the main treatment for T1DM; it can promote protein synthesis and inhibit protein breakdown (104). It should be noted that the dosages of these drugs need to be adjusted temporally according to a patient’s blood glucose levels. As mentioned above, insulin deficiency induces muscle protein degradation through the FoxO-dependent pathway. Therefore, blocking this pathway can prevent diabetic muscular atrophy, the complications of DM. Moreover, this could provide new insights into the prevention and treatment of diabetic muscular atrophy.
Anti-Inflammation and Antioxidation
There is positive feedback between inflammation and oxidative stress. Inflammation could induce cellular oxidative stress and oxidative stress also could lead inflammation (12, 73, 116). Aspirin is a non-steroidal anti-inflammatory drug that has been shown to alleviate insulin resistance and hyperglycemia in patients with T2DM by inhibiting the Janus kinase (JAK)/STAT and NF-κB signaling pathways (117). At the same time, aspirin also has good antioxidant properties in denervation induced muscle atrophy, as evidenced by reduced reactive oxygen species (66). However, the long-term use of nonsteroidal anti-inflammatory drugs is not recommended due to their adverse side effects. Other anti-inflammatory and antioxidant supplements, such as omega-3 fatty acids and vitamin D, represent viable options for long-term, daily usage (50). Omega-3 fatty acids, such as eicosapentaenoic and docosahexaenoic acids, are ingested through one’s diet. It is well known that inflammation and oxidative stress could be restricted by omega-3 fatty acids. Omega-3 fatty acids can ameliorate inflammation, reduce proinflammatory cytokine, inhibits free radicals (ROS) (107). They can reduce NF-κB activation by blocking the activation of Toll-like receptor 4 (TLR4) signaling (induced by lipopolysaccharides or saturated fatty acids). This subsequently exerts an anti-inflammatory effect (108). Higher ratios of omega-3 to omega-6 will lead to lower production of pro-inflammatory mediators derived from omega-6 (109). Omega-3 has also been shown to stimulate mTORC1 signaling, and thus could be used to repress muscle atrophy (110). Vitamin D, a ROS scavenger, can also act as an anti-inflammatory and antioxidant mediator (111). Pro-inflammatory cascades (i.e., NF-κB and TNF-α) have been shown to be downregulated when vitamin D binds to receptors in macrophages and lymphocytes (112). Vitamin D has also been shown to regulate muscle growth. In a muscle-specific vitamin D receptor knockout model, insulin resistance was found to occur, accompanied by the increased expression and activity of FOXO1. In vitamin D-treated muscle cells prepared in vitro, reductions were observed in the expression, nuclear translocation, and activity of FOXO1 (113). Therefore, vitamin D regulates muscle growth in part through FOXO1 signaling; it could be thereby used to prevent skeletal muscle atrophy.
In addition to regulating muscle atrophy, metformin and thiazolidinediones have relatively strong anti-inflammatory activities (115, 118). Therefore, incidences of muscle atrophy-related complications (or the degree of muscle atrophy) decrease when these drugs are used to treat DM. Systemic inflammation can lead to insulin resistance, whereas exercises can reduce systemic inflammatory markers and improve systemic and local muscle inflammation. Consequently, muscle atrophy following exercise can be relieved by increasing protein synthesis via the upregulation of the IRS1-PI3K-AKT pathway, the downregulation of the UPS, and the strengthening of mTORC2 activation (62). Overall, therefore, anti-inflammatory and antioxidant therapy may constitute an important strategy for combatting diabetic muscular atrophy.
Prospects
There is a growing understanding of the mechanism underlying DM-induced muscle atrophy. Insulin signaling plays a dominant role in controlling muscle size, and GC signaling significantly promotes muscle atrophy in patients with DM. In addition, inflammation, oxidative stress, and the activation of some signaling pathways can all also cause muscle atrophy in patients with DM. It is worth noting that inflammation is closely related to insulin resistance, insulin deficiency, and even oxidative stress regarding the mechanism of diabetic muscular atrophy. Therefore, regulating inflammation may represent another very important way to treat diabetic muscular atrophy, in addition to controlling insulin signaling. This could have important therapeutic implications for the treatment of diabetic muscular atrophy, and even DM. Oxidative stress is also an important cause of DM and its related complications, so inhibiting excessive ROS production is crucial for delaying the onset of DM. From the perspective of antioxidants, however, to date little progress has been made regarding the development of drugs for treating diabetic muscular atrophy. Protecting and restoring insulin function, reducing insulin resistance, and inhibiting inflammation through antioxidants all also represent viable research directions, as they could suppress diabetic muscular atrophy. Further in-depth research into the mechanism of diabetic muscular atrophy will contribute to the development of better treatments. A therapeutic method for diabetic muscular atrophy could potentially be developed through the co-regulation of insulin signaling, the GC signaling pathway, inflammation, and oxidative stress; this would be extremely valuable for improving the quality of life of patients with DM.
Author Contributions
(I) Conception and design: HS, MS and FX. (II) Administrative support: HS, MS and FX. (III) Collection and assembly of data: YS, ML, KW, GQ, H, WW, YJ, MC, CD. (IV) Data analysis and interpretation: YS, ML, KW, GQ, HL. (V) Manuscript writing: All authors. (VI) Final approval of manuscript: All authors.
Funding
This work was supported by the National Natural Science Foundation of China (Nos. 82072160, 81901933, 32130060), the Major Natural Science Research Projects in Universities of Jiangsu Province (No. 20KJA310012), the Natural Science Foundation of Jiangsu Province (Nos. BK20202013, BK20201209), the “QingLan Project” in Jiangsu Universities, the Priority Academic Program Development of Jiangsu Higher Education Institutions, Nantong Science and Technology Program (No. JC2021118).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
I am grateful for the contribution to language help and writing assistance from Lai Xu at Nantong University during the research.
References
- 1. Fujimaki S, Wakabayashi T, Takemasa T, Asashima M, Kuwabara T. Diabetes and Stem Cell Function. BioMed Res Int (2015) 2015:592915. doi: 10.1155/2015/592915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wang M, Tan Y, Shi Y, Wang X, Liao Z, Wei P. Diabetes and Sarcopenic Obesity: Pathogenesis, Diagnosis, and Treatments. Front Endocrinol (Lausanne) (2020) 11:568. doi: 10.3389/fendo.2020.00568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Nellaiappan K, Preeti K, Khatri DK, Singh SB. Diabetic Complications: An Update on Pathobiology and Therapeutic Strategies. Curr Diabetes Rev (2022) 18:e030821192146. doi: 10.2174/1573399817666210309104203 [DOI] [PubMed] [Google Scholar]
- 4. O'Neill BT, Bhardwaj G, Penniman CM, Krumpoch MT, Suarez Beltran PA, Klaus K, et al. Foxo Transcription Factors are Critical Regulators of Diabetes-Related Muscle Atrophy. Diabetes (2019) 68:556–70. doi: 10.2337/db18-0416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Yin L, Li N, Jia W, Wang N, Liang M, Yang X, et al. Skeletal Muscle Atrophy: From Mechanisms to Treatments. Pharmacol Res (2021) 172:105807. doi: 10.1016/j.phrs.2021.105807 [DOI] [PubMed] [Google Scholar]
- 6. Mahashabde M, Chaudhary G, Kanchi G, Rohatgi S, Rao P, Patil R, et al. An Unusual Case of Critical Illness Polyneuromyopathy. Indian J Crit Care Med (2020) 24:133–5. doi: 10.5005/jp-journals-10071-23346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Pohl C.A.-O.X., Dikic I. Cellular Quality Control by the Ubiquitin-Proteasome System and Autophagy. Science (2019) 366:818–22. doi: 10.1126/science.aax3769 [DOI] [PubMed] [Google Scholar]
- 8. Ribot C, Soler CA-O, Chartier A, Al Hayek SA-O, Naït-Saïdi R, Barbezier NA-O, et al. Activation of the Ubiquitin-Proteasome System Contributes to Oculopharyngeal Muscular Dystrophy Through Muscle Atrophy. PLoS Genet (2022) 18:e1010015. doi: 10.1371/journal.pgen.1010015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Milan G, Romanello V, Pescatore F, Armani A, Paik JH, Frasson L, et al. Regulation of Autophagy and the Ubiquitin-Proteasome System by the Foxo Transcriptional Network During Muscle Atrophy. Nat Commun (2015) 6:6670. doi: 10.1038/ncomms7670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Li Z, Cai B, Abdalla BA, Zhu X, Zheng M, Han P, et al. Lncirs1 Controls Muscle Atrophy via Sponging Mir-15 Family to Activate Igf1-Pi3k/Akt Pathway. J cachexia sarcopenia Muscle (2019) 10:391–410. doi: 10.1002/jcsm.12374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Yoshida TA-O, Delafontaine P. Mechanisms of Igf-1-Mediated Regulation of Skeletal Muscle Hypertrophy and Atrophy. Cells (2020) 9:1970. doi: 10.3390/cells9091970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wang W, Shen D, Zhang L, Ji Y, Xu L, Chen Z, et al. Skp-Sc-Evs Mitigate Denervated Muscle Atrophy by Inhibiting Oxidative Stress and Inflammation and Improving Microcirculation. Antioxidants (2022) 11:66. doi: 10.3390/antiox11010066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ferretti RA-O, Moura EA-O, Dos Santos VC, Caldeira EJ, Conte M, Matsumura CY, et al. High-Fat Diet Suppresses the Positive Effect of Creatine Supplementation on Skeletal Muscle Function by Reducing Protein Expression of Igf-Pi3k-Akt-Mtor Pathway. PLoS One (2018) 13:e0199728. doi: 10.1371/journal.pone.0199728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gonçalves DA-O, Silveira WA-O, Manfredi LH, Graça FA, Armani A, Bertaggia E, et al. Insulin/igf1 Signalling Mediates the Effects of β(2) -Adrenergic Agonist on Muscle Proteostasis and Growth. J Cachexia Sarcopenia Muscle (2019) 10:455–75. doi: 10.1002/jcsm.12395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Yadav A, Singh A, Phogat J, Dahuja A, Dabur R. Magnoflorine Prevent the Skeletal Muscle Atrophy via Akt/Mtor/Foxo Signal Pathway and Increase Slow-Myhc Production in Streptozotocin-Induced Diabetic Rats. J Ethnopharmacol (2021) 267:113510. doi: 10.1016/j.jep.2020.113510 [DOI] [PubMed] [Google Scholar]
- 16. Di Meo S, Iossa S, Venditti P. Skeletal Muscle Insulin Resistance: Role of Mitochondria and Other Ros Sources. J Endocrinol (2017) 233:R15–42. doi: 10.1530/JOE-16-0598 [DOI] [PubMed] [Google Scholar]
- 17. Aluganti Narasimhulu C, Singla DA-O. Amelioration of Diabetes-Induced Inflammation Mediated Pyroptosis, Sarcopenia, and Adverse Muscle Remodelling by Bone Morphogenetic Protein-7. J Cachexia Sarcopenia Muscle (2021) 12:403–20. doi: 10.1002/jcsm.12662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Al Mamun A, Rahman MM, Zaman S, Munira MS, Uddin MS, Rauf A, et al. Molecular Insight Into the Crosstalk of Ups Components and Alzheimer's Disease. Curr Protein Pept Sci (2020) 21:1193–201. doi: 10.2174/1389203721666200923153406 [DOI] [PubMed] [Google Scholar]
- 19. Wang W, Li M, Chen Z, Xu L, Chang M, Wang K, et al. Biogenesis and Function of Extracellular Vesicles in Pathophysiological Processes of Skeletal Muscle Atrophy. Biochem Pharmacol (2022) 198:114954. doi: 10.1016/j.bcp.2022.114954 [DOI] [PubMed] [Google Scholar]
- 20. Bilodeau PA, Coyne ES, Wing SS. The Ubiquitin Proteasome System in Atrophying Skeletal Muscle: Roles and Regulation. Am J Physiol Cell Physiol (2016) 311:C392–403. doi: 10.1152/ajpcell.00125.2016 [DOI] [PubMed] [Google Scholar]
- 21. Khalil R. Ubiquitin-Proteasome Pathway and Muscle Atrophy. Adv Exp Med Biol (2018) 1088:235–48. doi: 10.1007/978-981-13-1435-3_10 [DOI] [PubMed] [Google Scholar]
- 22. Park J, Cho J, Song EA-O. Ubiquitin-Proteasome System (Ups) as a Target for Anticancer Treatment. Arch Pharm Res (2020) 43:1144–61. doi: 10.1007/s12272-020-01281-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Jang SM, Redon CE, Thakur BL, Bahta MK, Aladjem MI. Regulation of Cell Cycle Drivers by Cullin-Ring Ubiquitin Ligases. Exp Mol Med (2020) 52:1637–51. doi: 10.1038/s12276-020-00508-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cai D, Frantz Jd, Tawa NE, Jr, Melendez PA, Oh B-C, Lidov HGW, et al. Ikkbeta/nf-Kappab Activation Causes Severe Muscle Wasting in Mice. Cell (2004) 119:285–98. doi: 10.1016/j.cell.2004.09.027 [DOI] [PubMed] [Google Scholar]
- 25. Sandri M, Gilbert A, Skurk C, Calabria E, Picard A, Walsh K, et al. Foxo Transcription Factors Induce the Atrophy-Related Ubiquitin Ligase Atrogin-1 and Cause Skeletal Muscle Atrophy. Cell (2004) 117:399–41. doi: 10.1016/S0092-8674(04)00400-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ma W, Cai Y, Shen Y, Chen X, Zhang L, Ji Y, et al. Hdac4 Knockdown Alleviates Denervation-Induced Muscle Atrophy by Inhibiting Myogenin-Dependent Atrogene Activation. Front Cell Neurosci (2021) 15:663384. doi: 10.3389/fncel.2021.663384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wang Y, Le WD. Autophagy and Ubiquitin-Proteasome System. Adv Exp Med Biol (2019) 1206:527–50. doi: 10.1007/978-981-15-0602-4_25 [DOI] [PubMed] [Google Scholar]
- 28. Kitajima Y, Yoshioka K, Suzuki N. The Ubiquitin-Proteasome System in Regulation of the Skeletal Muscle Homeostasis and Atrophy: From Basic Science to Disorders. J Physiol Sci (2020) 70:40. doi: 10.1186/s12576-020-00768-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Franco-Romero A, Sandri M. Role of Autophagy in Muscle Disease. Mol Aspects Med (2021) 82:101041. doi: 10.1016/j.mam.2021.101041 [DOI] [PubMed] [Google Scholar]
- 30. Zheng R, Huang S, Zhu J, Lin W, Xu H, Zheng X. Leucine Attenuates Muscle Atrophy and Autophagosome Formation by Activating Pi3k/Akt/Mtor Signaling Pathway in Rotator Cuff Tears. Cell Tissue Res (2019) 378:113–25. doi: 10.1007/s00441-019-03021-x [DOI] [PubMed] [Google Scholar]
- 31. Triolo M, Hood DA. Manifestations of Age on Autophagy, Mitophagy and Lysosomes in Skeletal Muscle. Cells (2021) 10:1054. doi: 10.3390/cells10051054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Castets P, Lin S, Rion N, Di Fulvio S, Romanino K, Guridi M, et al. Sustained Activation of Mtorc1 in Skeletal Muscle Inhibits Constitutive and Starvation-Induced Autophagy and Causes a Severe, Late-Onset Myopathy. Cell Metab (2013) 17:731–44. doi: 10.1016/j.cmet.2013.03.015 [DOI] [PubMed] [Google Scholar]
- 33. de Sire R, Rizzatti G, Ingravalle F, Pizzoferrato M, Petito V, Lopetuso L, et al. Skeletal Muscle-Gut Axis: Emerging Mechanisms of Sarcopenia for Intestinal and Extra Intestinal Diseases. Minerva Gastroenterol Dietol (2018) 64:351–62. doi: 10.23736/S1121-421X.18.02511-4 [DOI] [PubMed] [Google Scholar]
- 34. Mavalli MD, DiGirolamo DJ, Fan Y, Riddle RC, Campbell KS, van Groen T, et al. Distinct Growth Hormone Receptor Signaling Modes Regulate Skeletal Muscle Development and Insulin Sensitivity in Mice. J Clin Invest (2010) 120:4007–20. doi: 10.1172/JCI42447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Huang Z, Zhu J, Ma W, Sun H. Strategies and Potential Therapeutic Agents to Counter Skeletal Muscle Atrophy. Biotarget (2018) 2:8. doi: 10.21037/biotarget.2018.05.02 [DOI] [Google Scholar]
- 36. Yoon MS. Mtor as a Key Regulator in Maintaining Skeletal Muscle Mass. Front Physiol (2017) 8:788. doi: 10.3389/fphys.2017.00788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Mukund K, Subramaniam S. Skeletal Muscle: A Review of Molecular Structure and Function, in Health and Disease. Wiley Interdiscip Rev Syst Biol Med (2020) 12:21. doi: 10.1002/wsbm.1462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zheng LF, Chen PJ, Xiao WH. Signaling Pathways Controlling Skeletal Muscle Mass. Acta Physiologica Sinica (2019) 71:671–9. doi: 10.13294/j.aps.2019.0021 [DOI] [PubMed] [Google Scholar]
- 39. Rudar M, Naberhuis JK, Suryawan A, Nguyen HV, Stoll B, Style CC, et al. Prematurity Blunts the Insulin- and Amino Acid-Induced Stimulation of Translation Initiation and Protein Synthesis in Skeletal Muscle of Neonatal Pigs. Am J Physiol Endocrinol Metab (2021) 320:E551–65. doi: 10.1152/ajpendo.00203.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kim H, Cho SC, Jeong HJ, Lee HY, Jeong MH, Pyun JH, et al. Indoprofen Prevents Muscle Wasting in Aged Mice Through Activation of Pdk1/Akt Pathway. J Cachexia Sarcopenia Muscle (2020) 11:1070–88. doi: 10.1002/jcsm.12558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. de Proença ARG, Pereira KD, Meneguello L, Tamborlin L, Luchessi AA-O. Insulin Action on Protein Synthesis and its Association With Eif5a Expression and Hypusination. Mol Biol Rep (2019) 46:587–96. doi: 10.1007/s11033-018-4512-1 [DOI] [PubMed] [Google Scholar]
- 42. Ramos PA, Lytle KA, Delivanis D, Nielsen S, LeBrasseur NK, Jensen MD. Insulin-Stimulated Muscle Glucose Uptake and Insulin Signaling in Lean and Obese Humans. J Clin Endocrinol Metab (2021) 106:e1631–46. doi: 10.1210/clinem/dgaa919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. da Silva Rosa SC, Nayak N, Caymo AM, Gordon JW. Mechanisms of Muscle Insulin Resistance and the Cross-Talk With Liver and Adipose Tissue. Physiol Rep (2020) 8:e14607. doi: 10.14814/phy2.14607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Izzo A, Massimino E, Riccardi G, Della Pepa G. A Narrative Review on Sarcopenia in Type 2 Diabetes Mellitus: Prevalence and Associated Factors. Nutrients (2021) 13:183. doi: 10.3390/nu13010183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Tournadre A, Vial G, Capel F, Soubrier M, Boirie Y. Sarcopenia. Joint Bone Spine (2019) 86:309–14. doi: 10.1016/j.jbspin.2018.08.001 [DOI] [PubMed] [Google Scholar]
- 46. Cruz-Jentoft AJ, Sayer AA. Sarcopenia. Lancet (2019) 393:2636–46. doi: 10.1016/S0140-6736(19)31138-9 [DOI] [PubMed] [Google Scholar]
- 47. Mesinovic J, Zengin A, De Courten B, Ebeling PR, Scott D. Sarcopenia and Type 2 Diabetes Mellitus: A Bidirectional Relationship. Diabetes Metab Syndr Obes (2019) 12:1057–72. doi: 10.2147/DMSO.S186600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Czech MP. Insulin Action and Resistance in Obesity and Type 2 Diabetes. Nat Med (2017) 23:804–14. doi: 10.1038/nm.4350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Peng BY, Dubey NK, Mishra VK, Tsai FC, Dubey R, Deng W, et al. Addressing Stem Cell Therapeutic Approaches in Pathobiology of Diabetes and its Complications. J Diabetes Res (2018) 2018:7806435. doi: 10.1155/2018/7806435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Dalle S, Koppo K. Is Inflammatory Signaling Involved in Disease-Related Muscle Wasting? Evidence From Osteoarthritis, Chronic Obstructive Pulmonary Disease and Type Ii Diabetes. Exp Gerontol (2020) 137:110964. doi: 10.1016/j.exger.2020.110964 [DOI] [PubMed] [Google Scholar]
- 51. Tanaka M, Morifuji T, Yoshikawa M, Nakanishi R, Fujino H. Effects of Combined Treatment With Blood Flow Restriction and Low-Intensity Electrical Stimulation on Diabetes Mellitus-Associated Muscle Atrophy in Rats. J Diabetes (2019) 11:326–34. doi: 10.1111/1753-0407.12857 [DOI] [PubMed] [Google Scholar]
- 52. Essid SM, Bevington A, Brunskill NA-O. Proinsulin C-Peptide Enhances Cell Survival and Protects Against Simvastatin-Induced Myotoxicity in L6 Rat Myoblasts. Int J Mol Sci (2019) 20:1654. doi: 10.3390/ijms20071654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Bhardwaj G, Penniman CM, Jena J, Suarez Beltran PA, Foster C, Poro K, et al. Insulin and Igf-1 Receptors Regulate Complex I-Dependent Mitochondrial Bioenergetics and Supercomplexes via Foxos in Muscle. J Clin Invest (2021) 131:e146415. doi: 10.1172/jci146415lid-e146415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Uciechowski P, Dempke WCM. Interleukin-6: A Masterplayer in the Cytokine Network. Oncology (2020) 93:131–7. doi: 10.1159/000505099 [DOI] [PubMed] [Google Scholar]
- 55. Halim M, Halim A. The Effects of Inflammation, Aging and Oxidative Stress on the Pathogenesis of Diabetes Mellitus (Type 2 Diabetes). Diabetes Metab Syndr (2019) 13:1165–72. doi: 10.1016/j.dsx.2019.01.040 [DOI] [PubMed] [Google Scholar]
- 56. Forcina L, Miano C, Musarò A. The Physiopathologic Interplay Between Stem Cells and Tissue Niche in Muscle Regeneration and the Role of Il-6 on Muscle Homeostasis and Diseases. Cytokine Growth Factor Rev (2018) 41:1–9. doi: 10.1016/j.cytogfr.2018.05.001 [DOI] [PubMed] [Google Scholar]
- 57. Acharjee S, Ghosh B, Al-Dhubiab BE, Nair AB. Understanding Type 1 Diabetes: Etiology and Models. Can J Diabetes (2013) 37:269–76. doi: 10.1016/j.jcjd.2013.05.001 [DOI] [PubMed] [Google Scholar]
- 58. DeFronzo RA. Pathogenesis of Type 2 Diabetes Mellitus. Med Clin North Am (2004) 88:787–835. doi: 10.1016/j.mcna.2004.04.013 [DOI] [PubMed] [Google Scholar]
- 59. Akbari M, Hassan-Zadeh V. Il-6 Signalling Pathways and the Development of Type 2 Diabetes. Inflammopharmacology (2018) 26:685–98. doi: 10.1007/s10787-018-0458-0 [DOI] [PubMed] [Google Scholar]
- 60. Lauterbach MA, Wunderlich FT. Macrophage Function in Obesity-Induced Inflammation and Insulin Resistance. Pflugers Arch (2017) 469:385–96. doi: 10.1007/s00424-017-1955-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Akash MA-O, Rehman K, Liaqat A. Tumor Necrosis Factor-Alpha: Role in Development of Insulin Resistance and Pathogenesis of Type 2 Diabetes Mellitus. J Cell Biochem (2018) 119:105–10. doi: 10.1002/jcb.26174 [DOI] [PubMed] [Google Scholar]
- 62. Perry BD CM, Brennan-Speranza TC, Sbaraglia M, Jerums G, Garnham A, Wong C, et al. Muscle Atrophy in Patients With Type 2 Diabetes Mellitus: Roles of Inflammatory Pathways, Physical Activity and Exercise. Exerc Immunol Rev (2016) 22:94–109. [PMC free article] [PubMed] [Google Scholar]
- 63. Huang Z, Zhong L, Zhu J, Xu H, Ma W, Zhang L, et al. Inhibition of Il-6/Jak/Stat3 Pathway Rescues Denervation-Induced Skeletal Muscle Atrophy. Ann Trans Med (2020) 8:1681. doi: 10.21037/atm-20-7269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Madaro L, Passafaro M, Sala D, Etxaniz U, Lugarini F, Proietti D, et al. Denervation-Activated Stat3-Il-6 Signalling in Fibro-Adipogenic Progenitors Promotes Myofibres Atrophy and Fibrosis. Nat Cell Biol (2018) 20:917–27. doi: 10.1038/s41556-018-0151-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Zanders L, Kny M, Hahn A, Schmidt S, Wundersitz S, Todiras M, et al. Sepsis Induces Interleukin 6, Gp130/Jak2/Stat3, and Muscle Wasting. J Cachexia Sarcopenia Muscle (2022) 13:717–27. doi: 10.1002/jcsm.12867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Wan Q, Zhang L, Huang Z, Zhang H, Gu J, Xu H, et al. Aspirin Alleviates Denervation-Induced Muscle Atrophy via Regulating the Sirt1/Pgc-1alpha Axis and Stat3 Signaling. Ann Transl Med (2020) 8:1524. doi: 10.21037/atm-20-5460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Sun SC. The non-Canonical Nf-κb Pathway in Immunity and Inflammation. Nat Rev Immunol (2017) 17:545–58. doi: 10.1038/nri.2017.52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Mitchell JP, Carmody RJ. Nf-κb and the Transcriptional Control of Inflammation. Int Rev Cell Mol Biol (2018) 335:41–84. doi: 10.1016/bs.ircmb.2017.07.007 [DOI] [PubMed] [Google Scholar]
- 69. Thoma A, Lightfoot AP. Nf-Kb and Inflammatory Cytokine Signalling: Role in Skeletal Muscle Atrophy. Adv Exp Med Biol (2018) 1088:267–79. doi: 10.1007/978-981-13-1435-3_12 [DOI] [PubMed] [Google Scholar]
- 70. Ma W, Zhang R, Huang Z, Zhang Q, Xie X, Yang X, et al. Pqq Ameliorates Skeletal Muscle Atrophy, Mitophagy and Fiber Type Transition Induced by Denervation via Inhibition of the Inflammatory Signaling Pathways. Ann Trans Med (2019) 7:440. doi: 10.21037/atm.2019.08.101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Ma W, Xu T, Wang Y, Wu C, Wang L, Yang X, et al. The Role of Inflammatory Factors in Skeletal Muscle Injury. Biotarget (2018) 2:7. doi: 10.21037/biotarget.2018.04.01 [DOI] [Google Scholar]
- 72. Zhang L, Pan J, Dong Y, Tweardy DJ, Dong Y, Garibotto G, et al. Stat3 Activation Links a C/Ebpdelta to Myostatin Pathway to Stimulate Loss of Muscle Mass. Cell Metab (2013) 18:368–79. doi: 10.1016/j.cmet.2013.07.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Shen Y, Zhang R, Xu L, Wan Q, Zhu J, Gu J, et al. Microarray Analysis of Gene Expression Provides New Insights Into Denervation-Induced Skeletal Muscle Atrophy. Front Physiol (2019) 10:1298. doi: 10.3389/fphys.2019.01298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Zhang L, Du J, Hu Z, Han G, Delafontaine P, Garcia G, et al. Il-6 and Serum Amyloid a Synergy Mediates Angiotensin Ii-Induced Muscle Wasting. J Am Sci Nephrol (2009) 20:604–12. doi: 10.1681/ASN.2008060628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Wu C, Tang L, Ni X, Xu T, Fang Q, Xu L, et al. Salidroside Attenuates Denervation-Induced Skeletal Muscle Atrophy Through Negative Regulation of Pro-Inflammatory Cytokine. Front Physiol (2019) 10. doi: 10.3389/fphys.2019.00665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Juan CA, Pérez de la Lastra JM, Plou FJ, Pérez-Lebeña E. The Chemistry of Reactive Oxygen Species (Ros) Revisited: Outlining Their Role in Biological Macromolecules (DNA, Lipids and Proteins) and Induced Pathologies. Int J Mol Sci (2021) 22:4642. doi: 10.3390/ijms22094642-lid-4642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Luc K, Schramm-Luc A, Guzik TJ, Mikolajczyk TP. Oxidative Stress and Inflammatory Markers in Prediabetes and Diabetes. J Physiol Pharmacol (2019) 70:809–24. doi: 10.26402/jpp.2019.6.01 [DOI] [PubMed] [Google Scholar]
- 78. Keaney JF, Jr, Larson Mg, Vasan Rs, Wilson PWF, Lipinska I, Corey D, et al. Obesity and Systemic Oxidative Stress: Clinical Correlates of Oxidative Stress in the Framingham Study. Arterioscler Thromb Vasc Biol (2003) 23:434–9. doi: 10.1161/01.ATV.0000058402.34138.11 [DOI] [PubMed] [Google Scholar]
- 79. Guzik TJ, Skiba DS, Touyz RM, Harrison DG. The Role of Infiltrating Immune Cells in Dysfunctional Adipose Tissue. Cardiovasc Res (2017) 113:1009–23. doi: 10.1093/cvr/cvx108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Tan PL, Shavlakadze T, Grounds MD, Arthur PG. Differential Thiol Oxidation of the Signaling Proteins Akt, Pten or Pp2a Determines Whether Akt Phosphorylation is Enhanced or Inhibited by Oxidative Stress in C2c12 Myotubes Derived From Skeletal Muscle. Int J Biochem Cell Biol (2015) 62:72–9. doi: 10.1016/j.biocel.2015.02.015 [DOI] [PubMed] [Google Scholar]
- 81. Nikawa T, Ulla A, Sakakibara I. Polyphenols and Their Effects on Muscle Atrophy and Muscle Health. Molecules (2021) 26(16):4887. doi: 10.3390/molecules26164887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Panigrahy SK, Bhatt R, Kumar A. Reactive Oxygen Species: Sources, Consequences and Targeted Therapy in Type 2 Diabetes. J Drug Target (2017) 25:93–101. doi: 10.1080/1061186X.2016.1207650 [DOI] [PubMed] [Google Scholar]
- 83. Gerber PA, Rutter GA. The Role of Oxidative Stress and Hypoxia in Pancreatic Beta-Cell Dysfunction in Diabetes Mellitus. Antioxid Redox Signal (2017) 26:501–18. doi: 10.1089/ars.2016.6755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Rendra E, Riabov V, Mossel DM, Sevastyanova T, Harmsen MC, Kzhyshkowska J. Reactive Oxygen Species (Ros) in Macrophage Activation and Function in Diabetes. Immunobiology (2019) 224:242–53. doi: 10.1016/j.imbio.2018.11.010 [DOI] [PubMed] [Google Scholar]
- 85. Shen Y, Zhang Q, Huang Z, Zhu J, Qiu J, Ma W, et al. Isoquercitrin Delays Denervated Soleus Muscle Atrophy by Inhibiting Oxidative Stress and Inflammation. Front Physiol (2020) 11:988. doi: 10.3389/fphys.2020.00988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Ying W, Fu W, Lee YS, Olefsky JA-O. The Role of Macrophages in Obesity-Associated Islet Inflammation and β-Cell Abnormalities. Nat Rev Endocrinol (2020) 16:81–90. doi: 10.1038/s41574-019-0286-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Houstis N, Rosen Ed Fau - Lander ES, Lander ES. Reactive Oxygen Species Have a Causal Role in Multiple Forms of Insulin Resistance. Nature (2006) 440:944–8. doi: 10.1038/nature04634 [DOI] [PubMed] [Google Scholar]
- 88. Sharma VK, Singh TG. Chronic Stress and Diabetes Mellitus: Interwoven Pathologies. Curr Diabetes Rev (2020) 16:546–56. doi: 10.2174/1573399815666191111152248 [DOI] [PubMed] [Google Scholar]
- 89. Martín AI, Priego T, López-Calderón A. Hormones and Muscle Atrophy. Adv Exp Med Biol (2018) 1088:207–33. doi: 10.1007/978-981-13-1435-3_9 [DOI] [PubMed] [Google Scholar]
- 90. Beaupere C, Liboz A, Feve B, Blondeau B, Guillemain G. Molecular Mechanisms of Glucocorticoid-Induced Insulin Resistance. Int J Mol Sci (2021) 22:623. doi: 10.3390/ijms22020623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Fappi A, Neves JC, Kawasaki KA, Bacelar L, Sanches LN, da.Silva F,P, et al. Omega-3 Multiple Effects Increasing Glucocorticoid-Induced Muscle Atrophy: Autophagic, Ampk and Ups Mechanisms. Physiol Rep (2019) 7:1–18. doi: 10.14814/phy2.13966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Wang XJ, Xiao JJ, Liu L, Jiao HC, Lin H. Excessive Glucocorticoid-Induced Muscle Murf1 Overexpression is Independent of Akt/Foxo1 Pathway. Biosci Rep (2017) 37:BSR20171056. doi: 10.1042/BSR20171056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Sun H, Gong Y, Qiu J, Chen Y, Ding F, Zhao Q. Traf6 Inhibition Rescues Dexamethasone-Induced Muscle Atrophy. Int J Mol Sci (2014) 15:11126–41. doi: 10.3390/ijms150611126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Xie Y, Perry BD, Espinoza D, Zhang P, Price SR. Glucocorticoid-Induced Creb Activation and Myostatin Expression in C2c12 Myotubes Involves Phosphodiesterase-3/4 Signaling. Biochem Biophys Res Commun (2018) 503:1409–14. doi: 10.1016/j.bbrc.2018.07.056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Son YH, Jang EJ, Kim YW, Lee JH. Sulforaphane Prevents Dexamethasone-Induced Muscle Atrophy via Regulation of the Akt/Foxo1 Axis in C2c12 Myotubes. BioMed Pharmacother (2017) 95:1486–92. doi: 10.1016/j.biopha.2017.09.002 [DOI] [PubMed] [Google Scholar]
- 96. Cid-Díaz T, Leal-López S, Fernández-Barreiro F, González-Sánchez J, Santos-Zas I, Andrade-Bulos LJ, et al. Obestatin Signalling Counteracts Glucocorticoid-Induced Skeletal Muscle Atrophy via Nedd4/Klf15 Axis. J Cachexia Sarcopenia Muscle (2021) 12:493–505. doi: 10.1002/jcsm.12677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Takashima M, Ogawa W, Hayashi K, Inoue H, Kinoshita S, Okamoto Y, et al. Role of Klf15 in Regulation of Hepatic Gluconeogenesis and Metformin Action. Diabetes (2010) 59:1608–15. doi: 10.2337/db09-1679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Hain BA, Xu H, Waning DA-O. Loss of Redd1 Prevents Chemotherapy-Induced Muscle Atrophy and Weakness in Mice. J Cachexia Sarcopenia Muscle (2021) 12:1597–612. doi: 10.1002/jcsm.12795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Schiaffino S, Dyar KA, Ciciliot S, Blaauw B, Sandri M. Mechanisms Regulating Skeletal Muscle Growth and Atrophy. FEBS J (2013) 280:4294–314. doi: 10.1111/febs.12253 [DOI] [PubMed] [Google Scholar]
- 100. Hirata Y, Nomura K, Senga Y, Okada Y, Kobayashi K, Okamoto S, et al. Hyperglycemia Induces Skeletal Muscle Atrophy via a Wwp1/Klf15 Axis. JCI Insight (2019) 4:e124952. doi: 10.1172/jci.insight.124952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Kuramoto N, Nomura K, Kohno D, Kitamura T, Karsenty G, Hosooka T, et al. Role of Pdk1 in Skeletal Muscle Hypertrophy Induced by Mechanical Load. Sci Rep (2021) 11:3447. doi: 10.1038/s41598-021-83098-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Kalyani RR, Corriere M, Ferrucci L. Age-Related and Disease-Related Muscle Loss: The Effect of Diabetes, Obesity, and Other Diseases. Lancet Diabetes Endocrinol (2014) 2:819–29. doi: 10.1016/S2213-8587(14)70034-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Yang S, Yang G, Wu H, Kang L, Xiang J, Zheng P, et al. Microrna-193b Impairs Muscle Growth in Mouse Models of Type 2 Diabetes by Targeting the Pdk1/Akt Signalling Pathway. Diabetologia (2022) 65:563–81. doi: 10.1007/s00125-021-05616-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Kalaitzoglou E, Fowlkes JL, Popescu I, Thrailkill KM. Diabetes Pharmacotherapy and Effects on the Musculoskeletal System. Diabetes Metab Res Rev (2019) 35:e3100. doi: 10.1002/dmrr.3100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Kaneto HA-O, Kimura TA-O, Obata AA-O, Shimoda M, Kaku K. Multifaceted Mechanisms of Action of Metformin Which Have Been Unraveled One After Another in the Long History. Int J Mol Sci (2021) 22:2596. doi: 10.3390/ijms22052596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Peixoto LG, Teixeira RR, Vilela DD, Barbosa LN, Caixeta DC, Deconte SR, et al. Metformin Attenuates the Tlr4 Inflammatory Pathway in Skeletal Muscle of Diabetic Rats. Acta Diabetologica (2017) 54(10):943–51. doi: 10.1007/s00592-017-1027-5 [DOI] [PubMed] [Google Scholar]
- 107. Yakubu A, Azlan A, Loh SP, Md Noor S. Can Yellow Stripe Scad Compete With Salmon on its Role in Platelet Phospholipid Membrane and its Cardiovascular Benefits? J Obes (2019) 2019:4929131. doi: 10.1155/2019/4929131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Rogero MM, Calder PC. Obesity, Inflammation, Toll-Like Receptor 4 and Fatty Acids. Nutrients (2018) 10:432. doi: 10.3390/nu10040432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. DiNicolantonio JJ, O'Keefe JH. Importance of Maintaining a Low Omega-6/Omega-3 Ratio for Reducing Inflammation. Open Heart (2018) 5:3–6. doi: 10.1136/openhrt-2018-000946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Dupont J, Dedeyne L, Dalle S, Koppo K, Gielen E. The Role of Omega-3 in the Prevention and Treatment of Sarcopenia. Aging Clin Exp Res (2019) 31:825–36. doi: 10.1007/s40520-019-01146-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Lai TC, Chen YC, Cheng HH, Lee TL, Tsai JS, Lee IT, et al. Combined Exposure to Fine Particulate Matter and High Glucose Aggravates Endothelial Damage by Increasing Inflammation and Mitophagy: The Involvement of Vitamin D. Part Fibre Toxicol (2022) 19:25. doi: 10.1186/s12989-022-00462-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Kim DA-O, Meza CA-O, Clarke H, Kim JS, Hickner RC. Vitamin D and Endothelial Function. Nutrients (2020) 12:575. doi: 10.3390/nu12020575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Chen S, Villalta SA, Agrawal DK. Foxo1 Mediates Vitamin D Deficiency-Induced Insulin Resistance in Skeletal Muscle. J Bone Miner Res (2016) 31:585–95. doi: 10.1002/jbmr.2729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Seino SA-O, Sugawara K, Yokoi N, Takahashi H. B-Cell Signalling and Insulin Secretagogues: A Path for Improved Diabetes Therapy. Diabetes Obes Metab (2017) 19:22–9. doi: 10.1111/dom.12995 [DOI] [PubMed] [Google Scholar]
- 115. Nanjan MJ, Mohammed M, Prashantha Kumar BR, Chandrasekar MJN. Thiazolidinediones as Antidiabetic Agents: A Critical Review. Bioorg Chem (2018) 77:548–67. doi: 10.1016/j.bioorg.2018.02.009 [DOI] [PubMed] [Google Scholar]
- 116. Wang W, Shen D, Zhang L, Ji Y, Xu L, Chen Z, et al. Skp-Sc-Evs Mitigate Denervated Muscle Atrophy by Inhibiting Oxidative Stress and Inflammation and Improving Microcirculation. Antioxidants (Basel) (2021) 11(1):66. doi: 10.3390/antiox11010066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Bako HY, Ibrahim MA, Isah MS, Ibrahim S. Inhibition of Jak-Stat and Nf-κb Signalling Systems Could be a Novel Therapeutic Target Against Insulin Resistance and Type 2 Diabetes. Life Sci (2019) 239:117045. doi: 10.1016/j.lfs.2019.117045 [DOI] [PubMed] [Google Scholar]
- 118. Chang JE, Choi MS. A Molecular Perspective on the Potential Benefits of Metformin for the Treatment of Inflammatory Skin Disorders. Int J Mol Sci (2020) 21:8960. doi: 10.3390/ijms21238960 [DOI] [PMC free article] [PubMed] [Google Scholar]