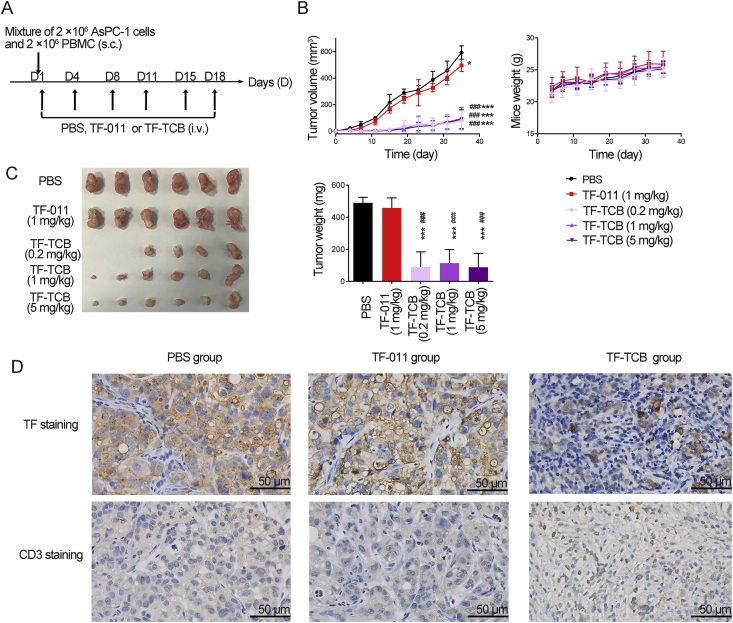
Figure 5.
Inhibition of tumor growth by TF-TCB in AsPC-1/PBMC co-grafting model. (A) Schematic depiction of tumor inoculation and treatment protocol. Female NOD/SCID mice (n = 6/group) were implanted subcutaneously (s.c.) with mixture of AsPC-1 cells (2 × 106) and PBMC (2 × 106) on Day 1, and administrated intravenously (i.v.) with PBS, TF-011 (1 mg/kg), or different doses of TF-TCB (0.2, 1 and 5 mg/kg) twice per week for a total of six doses. (B) Tumor volume and mice weight measured throughout the study. (C) Digital image and weight of the stripped tumors, two mice in the TF-TCB group (dose of 0.2 mg/kg) had no detected tumor when study completed. (D) Immunohistochemistry (IHC) analysis of tumors gained 6 days after first treatment. Tumors were stained for human TF and human CD3 (all brown) and were counterstained with hematoxylin (blue). Scale bar is indicated in each panel. Statistical analysis was based on two-tailed heteroscedastic t-test. ∗P < 0.05 and ∗∗∗P < 0.0001 vs. PBS group; ###P < 0.0001 vs. TF-011 group. Data are mean ± SD, n = 6.