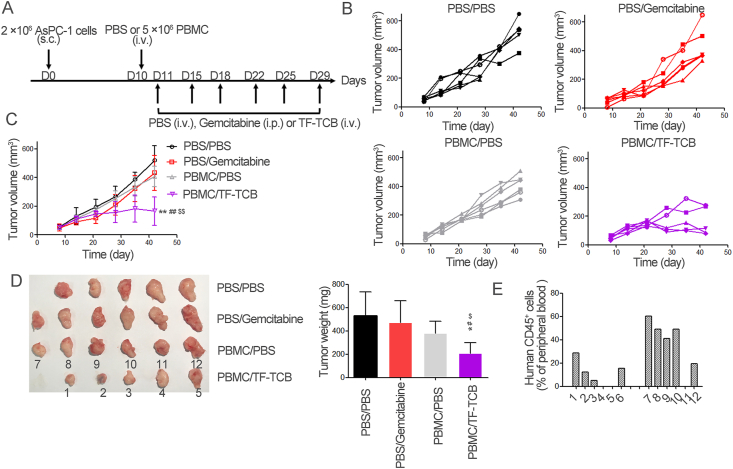
Figure 6.
Inhibition of tumor growth by TF-TCB in AsPC-1 xenograft model with intravenous transfer of PBMC. (A) Schematic depiction of tumor inoculation and treatment protocol. Female NOG mice were implanted s.c. with 2 × 106 AsPC-1 cells on Day 0. On Day 9, mice were divided into four groups (n = 6/group) based on tumor volume and 5 × 106 PBMC or PBS were injected i.v. into mice on Day 10. On Days 11–29, mice receiving PBMC were treated i.v. with PBS (PBMC/PBS group) and TF-TCB (5 mg/kg, PBMC/TF-TCB group), mice receiving PBS were treated intraperitoneally (i.p.) with PBS (PBS/PBS group) and gemcitabine (100 mg/kg, PBS/gemcitabine group). (B) Tumor volume of individual mice in each group measured throughout the study. (C) Tumor volume of the four groups. (D) Digital image and weight of the stripped tumors. There were only five tumors in the PBS/PBS and PBMC/TF-TCB group due to that one mouse in each group died before study ended. (E) Fourteen days after PBMC transfer, percentage of human CD45+ cells in peripheral blood of mice in PBMC/PBS and PBMC/TF-TCB group. 1–12 were mouse IDs, corresponding to tumor IDs in Fig. 6D. Statistical analysis was based on two-tailed heteroscedastic t-test. ∗P < 0.05 and ∗∗P < 0.01 vs. PBS/PBS group; #P < 0.05 and ##P < 0.01 vs. PBMC/PBS group; $P < 0.05 and $$P < 0.01 vs. PBS/gemcitabine group. Data are mean ± SD, n = 6.