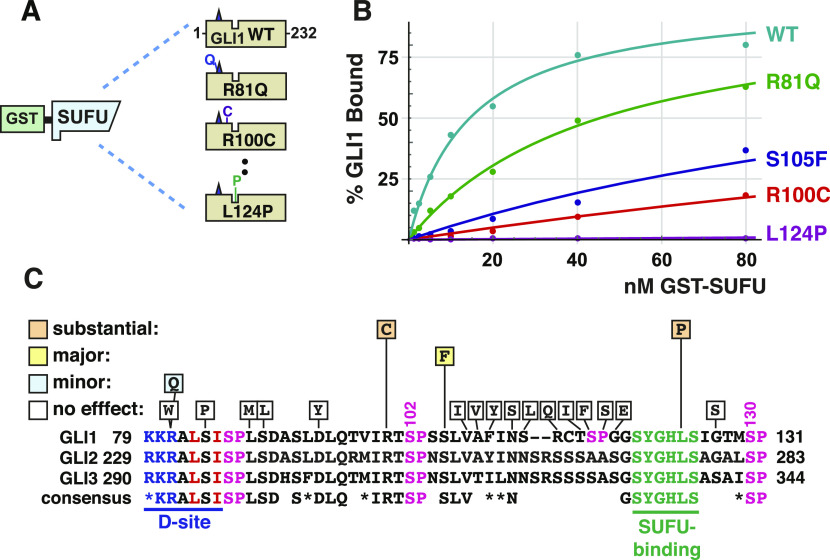
Figure 8. Effect of tumor-derived mutations on GLI1 binding to SUFU.
(A) Radiolabeled GLI11-232 variants were assessed for binding to GST-SUFU protein. (B) GLI1–SUFU binding isotherms of selected mutants. The data points on the graph are an average of three or four different experiments. (C) The chart shows which single substitutions in GLI11-232 had no effect (defined as an affinity change that was either less than twofold, and/or was not statistically significant), a minor effect (affinity decrease between two and fivefold that was statistically significant), a major effect (greater than 10-fold, significant), or a substantial effect (greater than 35-fold, significant). An alignment of human GLI1 residues 79–131 with the corresponding regions of human GLI2 and GLI3 is also shown, with the consensus shown below. Asterisks (*) indicate chemically similar residues that are also frequently substituted for one another in homologous sequences (Pearson, 1990).