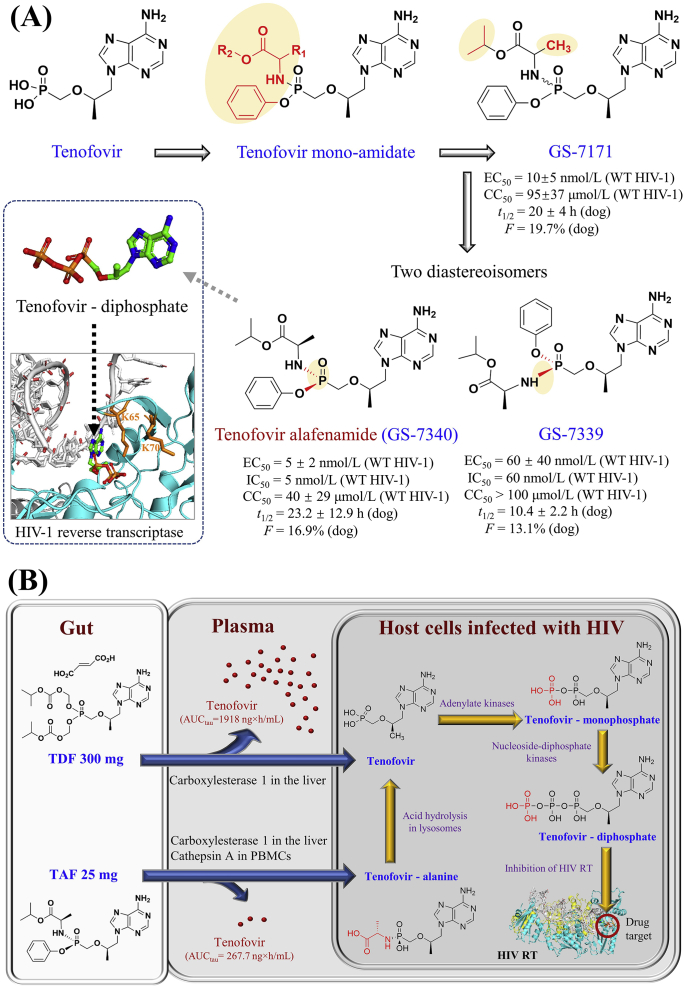
Figure 3.
Discovery and metabolic pathway of tenofovir alafenamide. (A) Discovery of tenofovir alafenamide. Anti-HIV-1 parameters and pharmacokinetic values are extracted from38. (B) Metabolic pathways of TDF 300 mg and TAF 25 mg from the gut to the blood plasma and lymphoid cells infected with HIV. At the steady-state, the plasma exposure of tenofovir at Day 10 was lower in the treatment of TAF 25 mg (AUCtau: 267.7 ng·h/mL) compared with TDF 300 mg (1918.0 ng·h/mL)43. TAF in blood enters primary hepatocytes and undertakes hydrolysis primarily by carboxylesterase one and cathepsin A that produce tenofovir–alanine conjugates within lymphocytes. TDF and TAF are converted to tenofovir and then phosphorylated to the intracellular active metabolite tenofovir diphosphate that blocks the catalytic site of HIV reverse transcriptase.