Abstract
Objective
Rare cases of genetically inherited atrioventricular block (AVB) have been reported; however, the heredity of AVB remains unknown. We aimed to assess the heredity of AVB.
Design, setting and participants
Using data from the Danish Civil Registration Registry, we established a nationwide cohort of individuals with parental links. Data were merged with information from the Danish Pacemaker and Implantable Cardioverter Defibrillator Registry, containing information on all pacemaker implantations performed in Denmark during the study period, to identify patients who received a first-time pacemaker because of AVB.
Results
A total of 4 648 204 individuals had parental links and a total of 26 880 consecutive patients received a first-time pacemaker due to AVB. Overall, the adjusted rate ratio (RR) of pacemaker implantation due to AVB was 2.1 (95% CI 1.8 to 2.5) if a father, mother or sibling had AVB compared with the risk in the general population. The adjusted RR was 2.2 (1.7–2.9) for offspring of mothers with AVB, 1.9 (1.5–2.4) for offspring of fathers with AVB and 3.5 (2.3–5.4) for siblings to a patient with AVB. The risk increased inversely proportionally with the age of the index case at the time of pacemaker implantation. The corresponding adjusted RRs were 15.8 (4.8–52.3) and 10.0 (3.3–30.4) if a mother or father, respectively, had a pacemaker implantation before 50 years.
Conclusion and relevance
First-degree relatives to a patient with AVB carry an increased risk of AVB with the risk being strongly inversely associated with the age of the index case at pacemaker implantation. These findings indicate a genetic component in the development of AVB in families with an early-onset disease.
Keywords: bradycardia; pacemaker, artificial
Introduction
Atrioventricular block (AVB) is the most common indication for pacemaker implantation, and the incidence of AVB increases with older age.1 Over the past decade, an average of 350 persons per million inhabitants have received a first-time pacemaker due to AVB in Denmark annually.1 In rare cases, AVB is found to be hereditary and may even be caused by a single genetic variant, for example, in genes coding for lamin A/C, titin, SCN5A or transthyretin amyloidosis.2–6 In most of these cases, AVB is only one component of several characteristics of the overall cardiac phenotype, whereas in other cases isolated genetically determined AVB is seen.7–9 However, in the majority of patients with AVB no aetiology of AVB is identified. One recent study examined the heredity of cardiac conduction defects including both sinus node dysfunction and AVB, and found an increased risk among first-degree family members.10 However, very little is known about the familial risk of AVB in the population. Therefore, we performed a nationwide study to assess the risk of AVB in first-degree relatives to patients with AVB.
Methods
Study population
This was a nationwide retrospective register-based cohort study. We used pacemaker implantation due to AVB as a surrogate marker for having clinically significant AVB. In Denmark, the indication for pacemaker implantation follows the European Society of Cardiology (ESC) guidelines on cardiac pacing.11 Using the Danish Pacemaker and Implantable Cardioverter Defibrillator (ICD) Registry, we identified all consecutive Danish patients receiving a first-time pacemaker on the indication of AVB in the period from 1 January 1982 to 9 May 2019. To study familial risk, we included only individuals from the Danish Civil Registration Registry and the Danish Pacemaker and ICD Registry for whom data on parental links were available in the Danish Civil Registration Registry.12 13 Also, patients who received a pacemaker after atrioventricular node ablation or as a result of complications to cardiac surgery or other cardiac intervention were excluded.
Registries
On time of birth or immigration, all people living in Denmark are given a unique and permanent civil registration number. This number is used in all national registries, which allows cross-linking of information between these registries.12 13 In the present study, we used data from the Danish Civil Registration Registry, the Danish National Patient Registry and the Danish Pacemaker and ICD Registry.
The Civil Registration Registry holds information on date of birth, sex, vital status, etc on all people living in Denmark. Moreover, data on parental links are available and are considered to have 100% coverage for individuals born in 1969 or later.12 In the period between 1950 and 1960, the percentage of individuals with parental links increased from 9.7% to 98.6%.12
The Danish National Patient Registry is a population-based administrative database that holds data on all hospital admissions and visits to outpatient clinics on an individual level. Diagnostic data in the Registry are exclusively given by treating physicians. Data were based on International Classification of Diseases, Eighth Revision (ICD-8) codes from the date the Registry was launched in 1977 until 1994. Thereafter, ICD-10 codes have been used.14
The Danish Pacemaker and ICD Registry is a national clinical database used by all implanting centres in Denmark. The implanting physician enters technical and clinical details of all device-related procedures prospectively into the database. The Registry holds information on all pacemaker implantations in Denmark from 1 January 1982 and onwards.
Familial risk
To investigate familial risk, we used data from the Civil Registration Registry and the Danish Pacemaker and ICD Registry to identify the following: (1) offspring of mothers who had received a pacemaker because of AVB, (2) offspring of fathers who had received a pacemaker because of AVB and (3) siblings to the first sibling in a sibship who received a pacemaker because of AVB. We aimed to investigate the risk of AVB as a function of age of the index person and with an a priori chosen specific cut-off of <50 years.
Baseline characteristics
Entry date for both index cases and relatives to the index case was defined as the day the index case received a first-time pacemaker. Cardiovascular comorbidity was identified using the Danish National Patient Registry. We considered patients to have congestive heart failure if a previous diagnosis of congestive heart failure was present (ICD-8 codes 425, 4270 or 4271 or ICD-10 codes DI110 or DI50). Diabetes mellitus was considered to be present with a previous diagnosis of diabetes mellitus (ICD-8 codes 2490 or 2500 or ICD-10 codes DE10, DE11, DE13 or DE14). Patients were considered to have suffered from a prior acute myocardial infarction (AMI) with a previous diagnosis of AMI (ICD-8 code 41 or ICD-10 codes DI21 or DI22). Atrial fibrillation (AF) or atrial flutter (AFL) was considered to be present if a patient had a previous diagnosis of AF or AFL (ICD-10 code DI48). Prior ventricular tachyarrhythmia (VT) was considered to be present with a previous diagnosis of VT (ICD-10 codes DI470, DI470C, DI472, DI472A, DI472B, DI472D or DI472H).
Statistical analysis
We used first-time pacemaker implantation due to AVB as a surrogate definition of AVB. The incidence rate of AVB was modelled as a function of age (intervals 0–4, 5–19, 20–24, 25–29, 30–34, 35–39, 40–44, 45–49, 50–54, 55–59, 60–64, 65–69, 70–74, 75–79, 80–84, 85–89 and >90 years), sex and calendar time intervals (<1985 and 5-year intervals thereafter until 2015).
In the first step of the analysis, we determined the incidence rate of AVB in the complete Danish population using Poisson regression. In a second step, the population rates were used as fixed reference rates. Subpopulations were constructed to assess if a family history of AVB was associated with an increase in AVB incidence as follows: for a maternal or a paternal history of AVB, all offspring were included in the subpopulation and their risk time started at the date of the AVB event of the affected parent and lasted until death, an event or end of follow-up, whichever came first (the maternal respectively paternal subpopulations). The reason not to start the risk time of the offspring from birth was to avoid conditioning on the future since this would carry a high risk of creating selection bias.15 For example, if parents have AVB at old age, one condition that the parents got old potentially creating a healthy selection bias among the children. For sibships, the first sibling (the index case) to have an event defined that history of AVB was present in the family (the sibships’ subpopulation). The rest of the siblings composed a subpopulation, and their risk time started at the date of the event of the first sibling. For each subpopulation, we used a Poisson model with the product of follow-up time and population reference rates as offset. The resulting RRs were reported with 95% CIs that were based on robust SEs taking into account the clustered data structure within families. For a maternal and for a paternal history of AVB, we also computed the RR if the parental index case was below or above 50 years of age and for sex of the offspring. In the adjust analyses for comorbidities, the incidence of AVB in the entire population was modelled as a function of age, sex, calendar time and comorbidity, where comorbidity was modelled as a time-depending variable (congestive heart failure, diabetes mellitus, prior AMI, AF/AFL and VT). The population reference rates were used as fixed rates in the Poisson regression. Statistical analyses were performed using STATA V.15.1 software.
Patient and public involvement
This was a nationwide retrospective register-based cohort study in which no individuals are identifiable.
Results
We identified 4 648 204 individuals with parental links from the Danish Civil Registration Registry. A total of 26 880 consecutive patients who received a first-time pacemaker due to AVB between 1 January 1982 and 9 May 2019 were identified from the Danish Pacemaker and ICD Registry. The sex- and age distribution among patients at the time of pacemaker implantation is displayed in figure 1. Of these, 311 patients were excluded because they were foreigners who received a temporary social security number while treated in a Danish hospital. Additionally, 25 cases were excluded because the social security number entered into the database was invalid.
Figure 1.
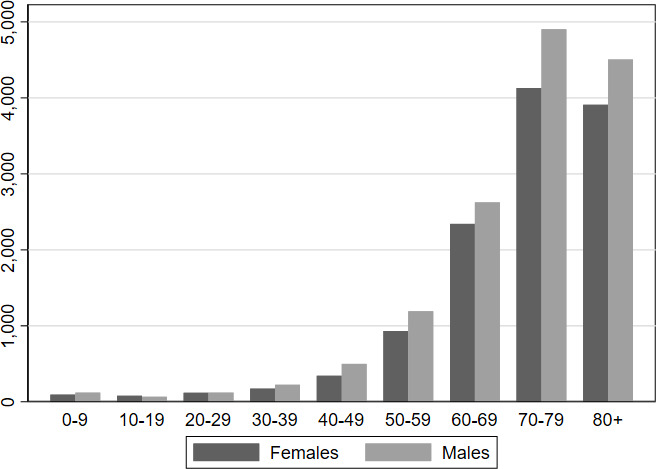
The sex- and age distribution among patients at the time of pacemaker implantation from the Danish Pacemaker and Implantable Cardioverter Defibrillator Registry.
There were 19 213 offspring of 9196 maternal index cases and 35 148 offspring of 16 050 paternal index cases. An overview of the patient distribution is shown in figure 2. Maternal index cases had a median age of 76.2 years (IQR 67.5–83.0 years), and paternal index cases had a median age of 75.1 years (IQR 67.6–81.6 years) at the time of pacemaker implantation. There were 2853 index siblings with 5349 siblings. Index siblings had a median age of 51.4 years (IQR 36.1–60.3) at the time of pacemaker implantation. Baseline characteristics for index cases are shown in table 1 and for relatives to the index case in table 2.
Figure 2.
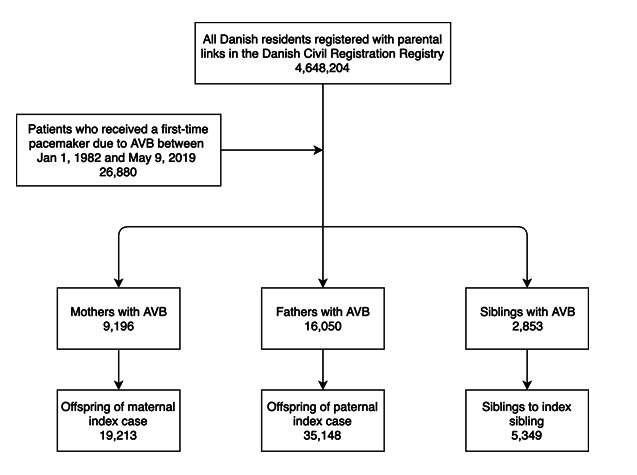
Flow chart of patient selection. Some mothers and fathers with atrioventricular block (AVB) also had siblings and consequently these individuals are registered as a parent as well as a sibling with AVB.
Table 1.
Baseline characteristics of index cases, that is, at the time of pacemaker implantation
| Index mother, n (%) | Index father, n (%) | Index sibling, n (%) | |
| Total | 9196 (100) | 16 050 (100) | 2853 (100) |
| Male sex | 0 (0) | 16 050 (100) | 1801 (63.1) |
| Median age | 76.2 (IQR 67.5–83.0) | 75.1 (IQR 67.6–81.6) | 51.4 (IQR 36.1–60.3) |
| Age at baseline, years | |||
| <30 | 126 | 80 | 539 |
| 30–40 | 130 | 180 | 299 |
| 40–50 | 286 | 399 | 497 |
| 50–60 | 695 | 1225 | 771 |
| 60–70 | 1596 | 3225 | 466 |
| 70–80 | 3032 | 5932 | 205 |
| 80–90 | 2821 | 4429 | 69 |
| >90 | 510 | 580 | 7 |
| Cardiovascular comorbidity | |||
| Congestive heart failure | 1084 (11.8) | 2469 (15.4) | 263 (9.2) |
| Diabetes mellitus | 1037 (11.3) | 2254 (14.0) | 211 (7.4) |
| Prior MI | 1380 (15.0) | 3650 (22.7) | 204 (7.2) |
| AF/AFL | 1387 (15.1) | 2899 (18.1) | 324 (11.4) |
| VT | 143 (1.6) | 297 (1.9) | 35 (1.2) |
AF, atrial fibrillation; AFL, atrial flutter; MI, myocardial infarction; VT, ventricular tachycardia.
Table 2.
Baseline characteristics of relatives to index cases, that is, at the time of pacemaker implantation in the relative
| Offspring of index mother, n (%) | Offspring of index father, n (%) | Sibling to index sibling, n (%) | |
| Total | 19 213 (100) | 35 148 (100) | 5349 (100) |
| Male sex | 10 220 (53.2) | 18 458 (52.5) | 2848 (53.2) |
| Median age | 46.9 (IQR 38.2–53.5) | 43.8 (IQR 35.6–50.4) | 48.8 (IQR 34.8–57.6) |
| Age at baseline, years | |||
| <30 | 2366 | 5278 | 1062 |
| 30–40 | 3203 | 7741 | 643 |
| 40–50 | 6254 | 12 870 | 1146 |
| 50–60 | 6048 | 8299 | 1497 |
| 60–70 | 1294 | 945 | 640 |
| 70–80 | 48 | 15 | 244 |
| 80–90 | 0 | 0 | 104 |
| >90 | 0 | 0 | 13 |
| Cardiovascular comorbidity | |||
| Congestive heart failure | 100 (1.1) | 115 (0.3) | 69 (1.3) |
| Diabetes mellitus | 177 (0.9) | 224 (0.6) | 88 (1.6) |
| Prior MI | 205 (1.1) | 211 (0.6) | 128 (2.4) |
| AF/AFL | 170 (0.9) | 218 (0.6) | 113 (2.1) |
| VT | 33 (0.2) | 36 (0.1) | 9 (0.2) |
AF, atrial fibrillation; AFL, atrial flutter; MI, myocardial infarction; VT, ventricular tachycardia.
Familial risk of AVB
Overall, compared with the general population, individuals with a father, mother or sibling who received a pacemaker due to AVB had an RR of 2.3 (95% CI 1.9 to 2.7) of developing AVB in a model adjusted for age, sex and calendar time (table 3). Further adjustment for baseline cardiovascular disease did not significantly alter this risk estimate (adjusted RR 2.1 (95% CI 1.8 to 2.5)). Offspring of mothers with AVB had an adjusted RR of 2.2 (95% CI 1.7 to 2.9) of developing AVB. No difference in risk based on sex of the offspring (p=0.56) was observed. Similarly, the adjusted RR among offspring of fathers with AVB was 1.9 (95% CI 1.5 to 2.4), and no difference in risk based on sex of the offspring was observed. Like for mothers, sex of the offspring did not affect the risk (p=0.79). Being sibling to a patient with AVB was associated with an adjusted RR of 3.5 (95% CI 2.3 to 5.4).
Table 3.
Rate ratio of atrioventricular block in first-degree relatives to index case
| Index case | Model 1 RR* | 95% CI | P-value | Model 2 RR† | 95% CI | P-value |
| Any father, mother or sibling | 2.3 | 1.9 to 2.7 | <0.001 | 2.1 | 1.8 to 2.5 | <0.001 |
| Any father, mother or sibling >50 years | 2.1 | 1.7 to 2.5 | <0.001 | 1.9 | 1.6 to 2.3 | <0.001 |
| Any father, mother or sibling <50 years | 9.0 | 5.5 to 14.9 | <0.001 | 8.0 | 4.8 to 13.4 | <0.001 |
| Mother | 2.4 | 1.9 to 3.2 | <0.001 | 2.2 | 1.7 to 2.9 | <0.001 |
| Mother >50 years | 2.3 | 1.8 to 3.0 | <0.001 | 2.1 | 1.6 to 2.8 | <0.001 |
| Mother <50 years | 16.6 | 5.0 to 55.1 | <0.001 | 15.8 | 4.8 to 52.3 | <0.001 |
| Daughters | 2.7 | 1.7 to 4.2 | <0.001 | 2.5 | 1.6 to 3.9 | <0.001 |
| Sons | 2.3 | 1.7 to 3.2 | <0.001 | 2.1 | 1.5 to 2.9 | <0.001 |
| Father | 2.0 | 1.6 to 2.5 | <0.001 | 1.9 | 1.5 to 2.4 | <0.001 |
| Father >50 years | 1.9 | 1.5 to 2.5 | <0.001 | 1.8 | 1.4 to 2.3 | <0.001 |
| Father <50 years | 10.5 | 3.4 to 32.1 | <0.001 | 10.0 | 3.3 to 30.4 | <0.001 |
| Daughters | 2.0 | 1.3 to 3.0 | <0.001 | 1.8 | 1.2 to 2.8 | <0.001 |
| Sons | 2.0 | 1.5 to 2.7 | <0.001 | 1.9 | 1.4 to 2.6 | <0.001 |
| Siblings | 3.7 | 2.5 to 5.6 | <0.001 | 3.5 | 2.3 to 5.4 | <0.001 |
*Adjusted for age, sex and calendar time (model 1).
†Adjusted for age, sex, calendar time, heart failure, diabetes, atrial fibrillation/flutter and myocardial infarction (model 2).
Age-related risk
As illustrated in table 3 and figure 3, the risk of AVB in relatives was strongly and inversely proportionally associated with the age of the index case. An additional analysis with risks reported below/after the age of 65 years is shown in online supplemental table 1. Accordingly, if the mother received her first pacemaker before the age of 50 years, the adjusted RR in her offspring was 15.8 (95% CI 4.8 to 52.3) as compared with 2.1 (95% CI 1.6 to 2.8) after the age of 50 years. In offspring of fathers who received a pacemaker before the age of 50 years, the adjusted RR was 10.0 (95% CI 3.3 to 30.4) as compared with 1.8 (95% CI 1.4 to 2.3) after the age of 50 years. As the age of the index case at the time of pacemaker implantation increased, the RR decreased towards one.
Figure 3.
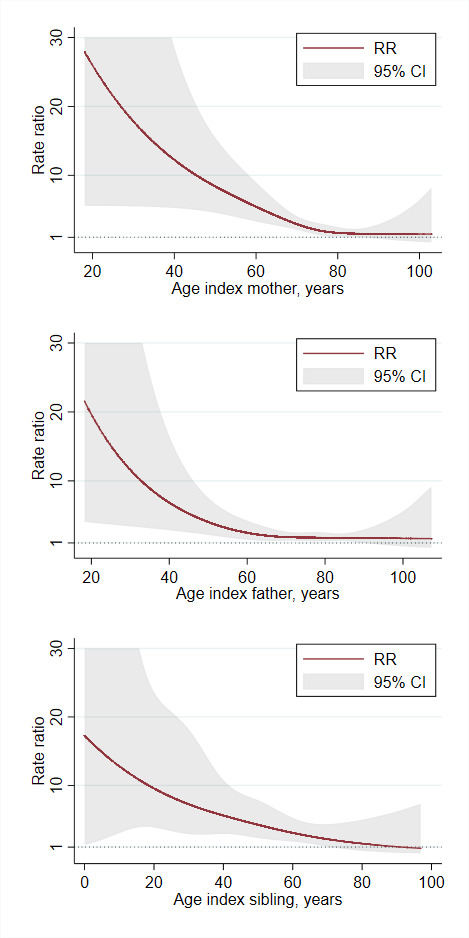
Adjusted rate ratio (RR) of atrioventricular block for first-degree relatives as function of age of the index relative.
heartjnl-2021-320411supp001.pdf (106KB, pdf)
Discussion
The present nationwide study showed that the risk of AVB in first-degree relatives to patients with AVB was more than doubled compared with the background population. This risk was inversely related to the age of the index case at pacemaker implantation and was increased to more than 10-fold in offspring of parents with pacemaker implantation before the age of 50 years. This finding suggests that younger patients with AVB requiring pacemaker implantation may have a genetically mediated disease, whereas occurrence of AVB at an older age can be a manifestation of several non-inherited conditions highly associated with old age.
One prior study has investigated familial clustering of cardiac conduction defects including AVB.10 In the Framingham Study they found a 1.7-fold increase in odds of pacemaker implantation among individuals with one or more first-degree relatives with a pacemaker.10 The study examined both sinus node dysfunction and AVB, and the study was limited by a moderate population size and the use of ICD codes on pacemaker implantations to define the outcome.10 These limitations have been overcome in the present study by using nationwide and highly credible data from the Danish Pacemaker and ICD Registry. Furthermore, our study adds to prior findings by demonstrating that familial risk increases exponentially the lower the age of the AVB onset, suggesting a strong genetic background in patients with early-onset disease.
An electrocardiographic PR interval measures atrioventricular node depolarisation and conduction, and an abnormal PR interval is closely related to AVB.16 In the 1980s, studies suggested a 34%–60% heritability of the PR interval.17 18 Currently, around 20 genes are known to be associated with AVB,7 but several additional genes associated with AVB may exist. A recent genome-wide association study in 92 000 European-descent individuals has identified 44 loci associated with the PR interval of which 34 were novel.19 Examination of these loci revealed that known and previously not-yet-reported biological processes were involved in atrioventricular conduction. These findings implicated developmental pathways and identified transcription factors, ion-channel genes and cell-junction/cell-signalling proteins in atrioventricular conduction as important causes of AVB.19 It is possible that these loci may also turn out to be of significance in patients with AVB in the presence of genetic variants with large effects on protein expression or function.
The finding of a genetic variation associated with a cardiac disease may have prognostic value. Our observation of a more than 10-fold higher risk in relatives to index cases with AVB before the age of 50 years suggests an underlying genetic cause of the disease in these families which leads to a number of considerations. First, these relatives may be at increased risk of sudden cardiac death due to sudden AVB and ventricular arrest. However, some genetic causes of AVB like laminopathies may also present with heart failure or malignant tachyarrhytmias.20 This may be an explanation for the fact that young patients with AVB have been shown to have a threefold to fourfold higher rate of death, heart failure or ventricular arrhythmias compared with the background population despite the implantation of a pacemaker.21 Therefore, our findings suggest that molecular genetic testing in these families as well as cardiac screening of relatives could possibly be of value to guide selection of the appropriate pacemaker device, initiation of preventive medical treatment, and thus prevention of disease development and sudden cardiac death. One recent study of 15 young patients with isolated AVB or sick sinus syndrome, and a suspicion of an inherited heart disease, found a pathogenic variant to be present in four patients in genes associated with either channelopathies or cardiomyopathies.22 However, further clinical studies evaluating the risk of sudden cardiac death in relatives of patients with AVB at an early age, as well as the clinical benefits of genetic testing or cardiac screening in this setting are needed to determine the clinical implications.
Strengths and limitations
The main strength of the study includes the use of well-validated registries that enabled us to identify almost complete nationwide data on family structure, comorbidities and pacemaker implantations due to AVB over a period of nearly four decades. Furthermore, the study was conducted within a setting characterised by a publicly paid healthcare system with equal and unlimited access for all citizens. However, some limitations need to be addressed. Information on baseline comorbidities was based on data from the Danish National Patient Registry. The validity of the cardiovascular diagnoses in this registry have proven to be high.23 However, diagnoses in the Danish National Patient Registry are limited to those given in relation to an admission to a hospital or a visit to an outpatient clinic, and consequently, diagnoses from general practices are not included. This leads to an underestimation of the proportion of patients suffering from, for example, diabetes in our population, since they are often treated in general practices.
In addition, awareness of heart disease treatable with cardiac pacing may have expedited diagnosis and treatment in relatives, thereby increasing the risk. It is, however, unlikely that a significant number of patients received a pacemaker without well-documented AVB; likewise, it is unlikely that many patients with AVB were not treated with cardiac pacing. In this context it is worth to note that the additional adjustment for baseline comorbidity did not significantly alter the risk ratios.
Conclusion
A family history of AVB is associated with an increased risk of AVB among first-degree relatives with the risk being strongly associated with age of the index case at pacemaker implantation. These findings indicate that genetic factors play a role in families with early-onset AVB.
Key messages.
What is already known on this subject?
The heredity of atrioventricular block (AVB) remains unknown although rare cases of genetically inherited AVB have been reported.
What might this study add?
In this study of 4 648 204 individuals, first-degree relatives to a patient with AVB carried an increased risk of AVB with the risk being strongly inversely associated with the age of the index case at pacemaker implantation.
How might this impact on clinical practice?
Whether the increased risk in these first-degree relatives is due to underlying, undiagnosed inherited heart disease presenting with the phenotype of AVB is unknown. Further clinical studies of the value of genetic testing in this setting and potential prognostic benefits are needed to determine the clinical implications.
heartjnl-2021-320411supp002.pdf (112.3KB, pdf)
Acknowledgments
We greatly appreciate the biostatistical support given by Professor Erik Parner, Department of Public Health—Department of Biostatistics, Aarhus University, Denmark.
Footnotes
Contributors: JRD, MKC and HKJ have planned, conducted and reported the work described in this article. JBJ, JCN and HB have participated in the conduct and report of the work described in this article. HKJ is responsible for the overall content as guarantor.
Funding: This work was supported by unrestricted research grants from Skibsreder Per Henriksen, R. og hustrus Foundation; the Novo Nordisk Foundation, Denmark (NNF18OC0031258) and A. P. Møller Foundation for the Advancement of Medical Science.
Competing interests: MKC has received lecture fees from Sanofi. JBJ reports personal fees from Medtronic, personal fees from Biotronik, outside the submitted work. JCN is supported by a grant from the Novo Nordisk Foundation, Denmark (NNF16OC0018658). HB is supported by the Innovation Fund Denmark (IFD) and NordForsk (through the funding to PM Heart (90580)). HKJ is supported by a grant from the Novo Nordisk Foundation, Denmark (NNF18OC0031258) and received lecture fees from Abbott, Denmark, and Biosense Webster, Europe.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
All data relevant to the study are included in the article or uploaded as supplementary information.
Ethics statements
Patient consent for publication
Not required.
Ethics approval
In Denmark, no ethical approval is needed for registry-based studies, in which no individuals are identifiable. The Danish study was approved by the Danish data protection agency (record no: 2016-051-000001).
References
- 1. 2015. DPaIRAr, 2015. Available: https://ssl.icddata.dk/download/Danish_Pacemaker_and_ICD_Register_Annual_Report_2015b.pdf
- 2. Kumar S, Baldinger SH, Gandjbakhch E, et al. Long-Term arrhythmic and Nonarrhythmic outcomes of lamin A/C mutation carriers. J Am Coll Cardiol 2016;68:2299–307. 10.1016/j.jacc.2016.08.058 [DOI] [PubMed] [Google Scholar]
- 3. Liu G, Yang Z, Chen W, et al. Novel missense variant in TTN cosegregating with familial atrioventricular block. Eur J Med Genet 2020;63:103752 10.1016/j.ejmg.2019.103752 [DOI] [PubMed] [Google Scholar]
- 4. Rudbeck-Resdal J, Nielsen JC, Bundgaard H, et al. Appropriate use of genetics in a young patient with atrioventricular block and family history of sudden cardiac death. HeartRhythm Case Rep 2019;5:169–72. 10.1016/j.hrcr.2018.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zaytseva AK, Karpushev AV, Kiselev AM, et al. Characterization of a novel SCN5A genetic variant A1294G associated with mixed clinical phenotype. Biochem Biophys Res Commun 2019;516:777–83 10.1016/j.bbrc.2019.06.080 [DOI] [PubMed] [Google Scholar]
- 6. Donnellan E, Wazni OM, Saliba WI, et al. Prevalence, incidence, and impact on mortality of conduction system disease in transthyretin cardiac amyloidosis. Am J Cardiol 2020;128:140–6 10.1016/j.amjcard.2020.05.021 [DOI] [PubMed] [Google Scholar]
- 7. Asatryan B, Medeiros-Domingo A. Molecular and genetic insights into progressive cardiac conduction disease. Europace 2019;21:1145–58 10.1093/europace/euz109 [DOI] [PubMed] [Google Scholar]
- 8. Rinné S, Ortiz-Bonnin B, Stallmeyer B, et al. POPDC2 a novel susceptibility gene for conduction disorders. J Mol Cell Cardiol 2020;145:74–83 10.1016/j.yjmcc.2020.06.005 [DOI] [PubMed] [Google Scholar]
- 9. Mastroianno S, Palumbo P, Castellana S, et al. Double missense mutations in cardiac myosin-binding protein C and myopalladin genes: a case report with diffuse coronary disease, complete atrioventricular block, and progression to dilated cardiomyopathy. Ann Noninvasive Electrocardiol 2020;25:e12687 10.1111/anec.12687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kaess BM, Andersson C, Duncan MS, et al. Familial clustering of cardiac conduction defects and pacemaker insertion. Circ Arrhythm Electrophysiol 2019;12:e007150 10.1161/CIRCEP.119.007150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Brignole M, Auricchio A, Baron-Esquivias G, et al. 2013 ESC guidelines on cardiac pacing and cardiac resynchronization therapy: the task force on cardiac pacing and resynchronization therapy of the European Society of Cardiology (ESC). developed in collaboration with the European heart rhythm association (EHRA). Eur Heart J 2013;34:2281–329 10.1093/eurheartj/eht150 [DOI] [PubMed] [Google Scholar]
- 12. Pedersen CB. The Danish civil registration system. Scand J Public Health 2011;39:22–5 10.1177/1403494810387965 [DOI] [PubMed] [Google Scholar]
- 13. Schmidt M, Pedersen L, Sørensen HT. The Danish civil registration system as a tool in epidemiology. Eur J Epidemiol 2014;29:541–9 10.1007/s10654-014-9930-3 [DOI] [PubMed] [Google Scholar]
- 14. Schmidt M, Schmidt SAJ, Sandegaard JL, et al. The Danish national patient registry: a review of content, data quality, and research potential. Clin Epidemiol 2015;7:449–90 10.2147/CLEP.S91125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Andersen PK, Keiding N. Interpretability and importance of functionals in competing risks and multistate models. Stat Med 2012;31:1074–88 10.1002/sim.4385 [DOI] [PubMed] [Google Scholar]
- 16. Arnolds DE, Chu A, McNally EM, et al. The emerging genetic landscape underlying cardiac conduction system function. Birth Defects Res A Clin Mol Teratol 2011;91:578–85 10.1002/bdra.20800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Havlik RJ, Garrison RJ, Fabsitz R, et al. Variability of heart rate, P-R, QRS and Q-T durations in twins. J Electrocardiol 1980;13:45–8 10.1016/S0022-0736(80)80008-2 [DOI] [PubMed] [Google Scholar]
- 18. Hanson B, Tuna N, Bouchard T, et al. Genetic factors in the electrocardiogram and heart rate of twins reared apart and together. Am J Cardiol 1989;63:606–9 10.1016/0002-9149(89)90907-7 [DOI] [PubMed] [Google Scholar]
- 19. van Setten J, Brody JA, Jamshidi Y, et al. Pr interval genome-wide association meta-analysis identifies 50 loci associated with atrial and atrioventricular electrical activity. Nat Commun 2018;9:2904 10.1038/s41467-018-04766-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Crasto S, My I, Di Pasquale E. The Broad Spectrum of LMNA Cardiac Diseases: From Molecular Mechanisms to Clinical Phenotype. Front Physiol 2020;11:761 10.3389/fphys.2020.00761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Dideriksen JR, Christiansen MK, Johansen JB, et al. Long-Term outcomes in young patients with atrioventricular block of unknown aetiology. Eur Heart J 2021;42:2060–8. 10.1093/eurheartj/ehab060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tassetti L, Girolami F, Fumagalli C, et al. Prevalence of inherited cardiac diseases among young patients requiring permanent pacing. Circ Arrhythm Electrophysiol 2021;14:e010562 10.1161/CIRCEP.121.010562 [DOI] [PubMed] [Google Scholar]
- 23. Sundbøll J, Adelborg K, Munch T, et al. Positive predictive value of cardiovascular diagnoses in the Danish national patient registry: a validation study. BMJ Open 2016;6:e012832 10.1136/bmjopen-2016-012832 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
heartjnl-2021-320411supp001.pdf (106KB, pdf)
heartjnl-2021-320411supp002.pdf (112.3KB, pdf)
Data Availability Statement
All data relevant to the study are included in the article or uploaded as supplementary information.


