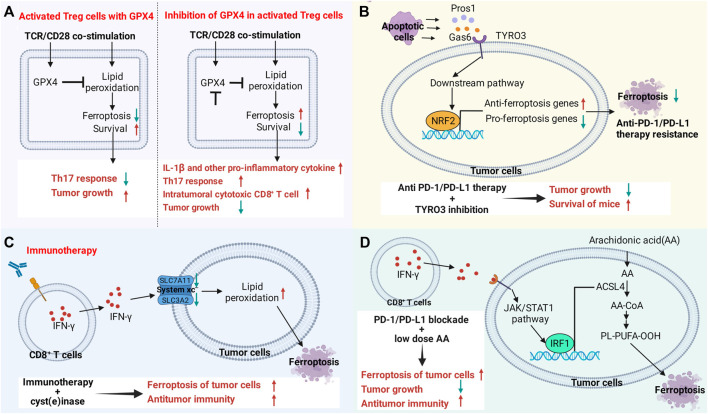
FIGURE 4.
Targeting the ferroptosis pathway in immune cells or cancer cells reverses immunotherapy resistance or enhances therapeutic efficacy. (A) The ferroptosis signaling pathway in immune cells regulates antitumor immune function. Gpx4 protects activated Treg cells from lipid peroxidation and ferroptosis. Loss of Gpx4 leads to excessive accumulation of lipid peroxides and ferroptosis of Treg cells after TCR/CD28 co-stimulation. Gpx4-deficient Treg cells upregulate the production of IL-1β and TH17 responses, increasing the number and killing activity of intratumoral CD8+ T cells. Knockdown of Gpx4 in Treg cells inhibited tumor growth and simultaneously enhanced antitumor immunity. (B) TYRO3 expressed by tumor cells leads to resistance to anti-PD-1/PD-L1 therapy by inhibiting tumor ferroptosis. Some molecules produced by apoptotic cells in the tumor microenvironment activate the AKT/NRF2 axis after binding to TYRO3, thereby promoting the transcription of ferroptosis-inducing genes and inhibiting the expression of ferroptosis-inducing genes, leading to anti-PD-1/PD-L1 therapy resistance. Inhibition of TYRO3 promotes tumor ferroptosis and sensitizes resistant tumors to anti-PD-1 therapy. (C,D) CD8+ T cell-derived IFN-γ in the tumor microenvironment promotes lipid peroxidation and ferroptosis in tumor cells. Drugs that promote ferroptosis enhance the antitumor efficacy of immunotherapy. (C) IFN-γ promotes lipid peroxidation and ferroptosis in tumor cells by inhibiting the expression of SLC3A2 and SLC7A11. (D) IFN-γ activates the JAK/STAT1 signaling pathway in tumor cells, which in turn promotes the expression of ACSL4 through interferon regulatory factor 1 (IRF1). Supplementation with low-dose AA promotes ferroptosis in tumor cells and enhances the antitumor activity of checkpoint therapy.