Abstract
Articular cartilage is composed of chondrocytes surrounded by a porous permeable extracellular matrix. It has a limited spontaneous healing capability post-injury which, if left untreated, can result in severe osteochondral disease. Currently, osteochondral (OC) defects are treated by bone marrow stimulation, artificial joint replacement, or transplantation of bone, cartilage, and periosteum, while autologous osteochondral transplantation is also an option; it carries the risk of donor site damage and is limited only to the treatment of small defects. Allografts may be used for larger defects; however, they have the potential to elicit an immune response. A possible alternative solution to treat osteochondral diseases involves the use of stromal/stem cells. Human adipose-derived stromal/stem cells (ASCs) can differentiate into cartilage and bone cells. The ASC can be combined with both natural and synthetic scaffolds to support cell delivery, growth, proliferation, migration, and differentiation. Combinations of both types of scaffolds along with ASCs and/or growth factors have shown promising results for the treatment of OC defects based on in vitro and in vivo experiments. Indeed, these findings have translated to several active clinical trials testing the use of ASC-scaffold composites on human subjects. The current review critically examines the literature describing ASC-scaffold composites as a potential alternative to conventional therapies for OC tissue regeneration.
Keywords: adipose-derived stem cells, osteochondral defects, stem cells, scaffold, tissue engineering
1 Introduction
Tissue engineering is an interdisciplinary field which amalgamates the applications and principles of life sciences and engineering to develop biological substitutes to maintain, improve, or restore tissue function (Langer and Vacanti, 2016). While the body generally has good self-healing potential, the extent of self-repair varies among different tissues and may also be affected by diseases or injuries (Lanza et al., 2020). Tissue engineering involves the use of cells, scaffolds, and/or bioactive molecules to integrate and perform tissue repair (Lee et al., 2014). A substantial challenge associated with the implantation of cells alone into the body is the uncertainty of cellular fate post-implantation. Unlike drugs whose actions can be correlated with the physiological response, for stem cells, there is a need to track, quantify, and check the viability of the cells at the desired site of action (Nguyen et al., 2016). Moreover, there is a loss of implanted cells from the site of action due to systemic resorption and damage by the inflammatory microenvironment (Afessa and Peters, 2006). Therefore, to enhance the retention and viability of cells at the site of tissue injury, scaffolds and biological factors serve as useful adjuvants to cell therapy alone. Scaffolds can be broadly divided into two categories, either natural or synthetic. Regardless of their origin, scaffolds are intended to support the cell’s attachment, proliferation, migration, and differentiation (Keane and Badylak, 2014). Currently, there are multiple clinically relevant biomaterials for tissue engineering applications of skin, cartilage, bone, and heart available in the market (Lee et al., 2014).
The present article is intended to provide an overview of the recent literature focused on the application of adipose-derived stromal/stem cells (ASCs) and scaffold composites for the treatment of OC defects. The search terms used to review the primary literature were “adipose-derived stem cells,” “osteochondral defects,” “scaffold,” and “hydrogel.” Studies were included if the ASC-seeded scaffolds were evaluated in the context of OC defect regeneration; in contrast, those studies conducted only on bone or cartilage defects were excluded.
2 Osteochondral Defects
The damage to the articular cartilage and the underlying subchondral bone leads to defects known as OC defects. These defects can result from aging, physical trauma, or chronic diseases such as osteochondritis or osteoarthritis (OA) (Hunter et al., 2014). While investigators initially believed that OA only adversely affected the articular cartilage (AC), it is now established that OA causes damage to all tissues within the diarthrodial joint, including ligaments, joint capsule, menisci, subchondral bone, and synovial membrane (Torres et al., 2006; Krasnokutsky et al., 2011). The impaired crosstalk between the AC and subchondral bone is a complex phenomenon, capable of inducing adverse biochemical and biomechanical changes in the osteochondral region (Hu et al., 2020). Such changes can result in the development of diseases including osteosclerosis, osteonecrosis, and osteochondritis in the subchondral regions. The histological changes that appear in the subchondral bone are a consequence of impaired bone mineralization and turnover, thus reducing overall bone density and subchondral bone volume. Moreover, these effects lead to alteration in the biomechanical properties of the osteochondral unit, thereby reducing the load-bearing capability of the osteochondral unit. The exact cause of OC defects is yet to be determined; however, it is generally believed that abnormal endochondral ossification, genetic factors, and repetitive microtrauma play a significant role in disease progression (Grimm et al., 2014). Furthermore, some studies have suggested that either repetitive stress on the osteochondral unit or a single acute event can trigger AC degradation via the expression of proteolytic enzymes. Induced expression of disintegrin and metalloproteinase with thrombospondin motifs (ADAMTS) and matrix metalloproteinase (MMP) family proteins can degrade the matrix composition, thereby contributing to the disease pathogenesis (Buckwalter et al., 2013; Chang et al., 2019).
The AC is a porous, avascular, and aneural structural organization composed of chondrocytes surrounded by an extracellular matrix (ECM) containing proteoglycans and collagen type II (Clouet et al., 2009). The organization and composition of the ECM control the mechanical properties of the AC (Sanchez-Adams et al., 2014; Prein et al., 2016). Because of the avascular nature of the AC, it lacks the ability to heal spontaneously when injured. In the United States and Europe, two million people are diagnosed with AC defects annually (Mumme et al., 2016). Nevertheless, there are no effective treatments available for AC injuries (Boyer et al., 2020).
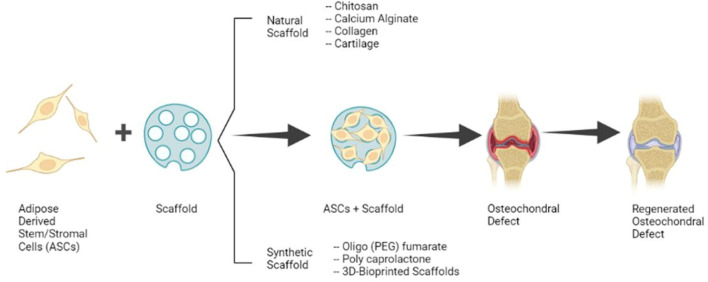
The present standard of care for OC defects includes microfracture which often results in the formation of fibrocartilage and is unsuitable for the treatment of large defects (more than 2–4 cm2). Possible therapy for large defects is the OC autograft transfer system which results in better healing; however, this procedure is technically difficult, has a risk of donor site morbidity, and the transplanted cartilage may fail to integrate. While autologous chondrocyte implantation has shown satisfactory results in the repair of hyaline-like cartilage, it is expensive and laborious because of the need for chondrocyte isolation and expansion in vitro (Chimutengwende-Gordon et al., 2020). Because of the limitations of these established therapies, recent studies have concentrated on the clinical translation of ASCs and scaffolds alone or as composites for the regeneration of OC defects (Figure 1).
FIGURE 1.
Clinical application of ASCs and Scaffolds for the Regeneration of OC Defects. (Image created with Biorender.com).
3 Adipose-Derived Stromal/Stem Cells
Adipose tissue is present in humans diffusely throughout the body with major depots located in the abdomen, buttocks, thighs, arms, and breasts within both subcutaneous and intraperitoneal compartments. The main function associated with adipose tissue historically has been to store excess energy in the form of triglycerides within adipocytes. There has been an increased appreciation of adipose tissue as an endocrine organ since it secretes adipokines which modulate appetite and insulin sensitivity (Rosen and Spiegelman, 2014). In addition, adipose tissue regulates the body temperature and glucose and lipid homeostasis (Seale et al., 2011; Kajimura et al., 2015). There are two main types of adipose tissues, the white and beige/brown adipose tissue; however, in tissue engineering and regenerative medicine, the white adipose tissue is used more frequently because of its greater availability in the adult human population (Minteer et al., 2012). The ASCs isolated from white adipose tissue have the potential to differentiate into a variety of cell types including adipocytes, osteoblasts, chondrocytes, cardiac myocytes, and skeletal myocytes (Zuk et al., 2001; Lin et al., 2006; Estes et al., 2010; Boyer et al., 2020). In addition, the ASCs have been extensively investigated as adult stromal/stem cells for cartilage (Estes et al., 2010) and bone tissue-engineering applications (Rada et al., 2011). In addition to their differentiation capability, ASCs have a low immunological reactivity due to the low expression or absence of immunogenic surface antigens including cluster of differentiation (CD) 40, CD40L, CD80, CD86, and major histocompatibility complex (MHC) II (Bourin et al., 2013). The ASCs retain their low immunological reactivity even after osteogenic differentiation (Liu et al., 2013).
3.1 Source of Adipose-Derived Stromal/Stem Cells
Subcutaneous adipose tissue of arms, thighs, and abdomen are the most clinically relevant sources of ASCs (Bacakova et al., 2018; Louwen et al., 2018). The proliferation and differentiation of ASCs isolated from different physiological depots can vary (Fraser et al., 2007). For example, ASCs isolated from the medial thigh, trochanteric, or superficial abdominal regions and upper thigh show higher apoptosis than ASCs isolated from superficial abdominal regions (Schipper et al., 2008). It has been also reported that the isolation procedures can impact the ASC’s plasticity, functionality, and quality (Argentati et al., 2018; Palumbo et al., 2018). The main function of white adipose tissue is the storage of excess energy in the form of triglycerides and is found in visceral and subcutaneous adipose tissues (Himms-Hagen et al., 2000; Barbatelli et al., 2010). In terms of yield, the highest number of ASCs is obtained from arm adipose tissue while the highest plasticity is found in the white inguinal adipose tissue. In adults, brown adipose tissue is less abundant than white adipose tissues; however, brown adipose tissues can be found in neonates in the area of the neck, mediastinum, and interscapular tissues. In contrast to white adipose tissues, the primary function of beige/brown adipose tissues is thermogenesis or non-shivering heat generation. Consequently, ASCs derived from beige/brown adipose tissue display distinct characteristics compared with ASCs derived from white adipose tissue, including the ability to undergo skeletal myogenic differentiation (Raposio and Bertozzi, 2017).
3.2 Differentiation Capability of Adipose-Derived Stromal/Stem Cells
In tissue engineering and regenerative medicine, ASCs have significant utility because of their capability to differentiate along multiple lineages. The ASCs can efficiently differentiate into mesenchymal lineages such as bone, fat, and cartilage when exposed in vitro to appropriate inductive culture conditions. This is consistent with their ability to regenerate bone, fat, and cartilage in vivo. While there have been reports of ASC differentiation along non-mesodermal lineages (neurons, cardiomyocytes, and hepatocytes), the efficiency of non-mesodermal lineage ASC differentiation in vivo remains less well documented (Bunnell, 2021).
Compared with bone marrow-derived mesenchymal stem cells (BMSC), ASCs are considered inferior in the case of osteogenic differentiation in vitro (Brennan et al., 2017). However, it is well-established that ASCs undergo osteogenic differentiation both in vitro (Girolamo et al., 2007; Kuterbekov et al., 2018) and in vivo (Supronowicz et al., 2011; Schubert et al., 2013; Lo et al., 2017). Moreover, the differentiation of ASCs into bone and cartilage has also exhibited promising results in clinical applications (Sándor, 2012; Vériter et al., 2015; Farré-Guasch et al., 2018). It has been observed that the OC differentiation of ASCs can be enhanced by exogenous biological factors. The addition of growth factors such as transforming growth factor β1 (TGF-β1), TGF-β3 (Fan et al., 2010), and bone morphogenic protein 4 (BMP4) (Lópiz-Morales et al., 2010) enhance the OC differentiation of ASCs. It has also been shown in pre-clinical studies that the activation of fibroblast growth factor (FGF2) signaling enhances MSC-mediated cartilage repair (Grafe et al., 2018). Furthermore, additional studies have identified a correlation between cell culture oxygen levels and the differentiation and proliferation of ASCs. Generally, native chondrocytes and cartilage are cultured in low oxygen conditions ranging from 2–7% saturation, comparable with oxygen levels experienced by cartilage in vivo (Zhou et al., 2004). Consistent with these observations, ASCs display enhanced chondrogenesis when cultured in vitro under 5% oxygen (Merceron et al., 2010; Weijers et al., 2011).
4 Scaffolds in Tissue Engineering
In the field of tissue engineering, scaffolds serve as a fundamental component by virtue of their biochemical and mechanical properties. The scaffold composition and morphology influence cell adhesion, migration, proliferation, and differentiation (Litowczenko et al., 2021). Ideal scaffolds should mimic the natural environment, such that their pore size, surface area, porosity, and mechanical properties closely resemble those of the target tissue (Silva et al., 2007; Nava et al., 2016). In addition, scaffolds should be degradable and biocompatible (Gomes and Reis, 2004) with minimal risk of cytotoxicity or genotoxicity (Cvetković et al., 2018). Furthermore, ideal scaffolds should support attachment and proliferation by a wide range of cell types. It is desirable that the scaffolds are suitable for advanced functions such as encapsulation and release of bioactive factors, for example, growth factors, anti-inflammatory agents, and anti-bacterial molecules. Newly developed scaffolds with these versatile characteristics are showing promise in a variety of tissue engineering applications (Litowczenko et al., 2021) including the regeneration of the heart (Fang et al., 2020), skin (Khan et al., 2018; Maged et al., 2019), nervous tissues (Kuzmenko et al., 2018; Sadeghi et al., 2019), and bone tissue (Mohiuddin et al., 2019a; Zhang et al., 2019).
4.1 Scaffolds for Osteochondral Defect Regeneration
An optimal scaffold for OC regeneration should have the ability to support osteogenesis, chondrogenesis, and angiogenesis, and have space for nutrient and cell infiltration (Roddy et al., 2018). Scaffolds based on polymers, ceramics, and decellularized ECM have shown promising results so far for the regeneration of OC defects (Ghassemi et al., 2018; Roddy et al., 2018). The shape, size, total porosity, and pore interconnectivity significantly influence the therapeutic effect of a scaffold (Yamane et al., 2007). The pore structure is a critical feature among these properties since it is the literal “gatekeeper” of cell migration, growth, and nutrient flow (Zeltinger et al., 2001). Pore sizes that are too small will restrict cell migration, nutrient diffusion and waste removal and adversely affect cell viability, whereas pore sizes that are too large can diminish cell attachment and biomechanical stiffness of the scaffold (Karageorgiou and Kaplan, 2005). Presently, the two types of scaffolds used in bone and cartilage tissue engineering are synthetic and natural scaffolds.
4.1.1 Synthetic Scaffolds
The most widely used synthetic scaffolds for OC regeneration include polylactide (PLA), polyglycolic acid (PGA), polylactic-co-glycolic acid (PLGA), poly-L-lactide (PLLA), polyethylene glycol (PEG), polyvinyl alcohol (PVA), and polycaprolactone (PCL). They are prepared as hydrogels or nanofibrous scaffolds (Sánchez-Téllez et al., 2017; Yang et al., 2017; Dai et al., 2018; Kudva et al., 2018; Critchley et al., 2020) which can be optimized for load-bearing tissues by adjusting their mechanical strength through varying concentrations and processing methods (Grigore, 2017). The stiffness of synthetic scaffolds is not only necessary for maintaining structural integrity and load-bearing in vivo, but it also provides physical cues for stem cells to differentiate along the OC lineage (Davis et al., 2021). One of the limitations of synthetic scaffolds is that they have a relatively low affinity for cell attachment compared to natural scaffolds. Therefore, to overcome this problem, different types of bioactive materials such as growth factors and/or peptides are added to improve cell attachment (Woodard and Grunlan, 2018). Among synthetic scaffolds, PEG is widely used because of its biocompatibility, hydrophilicity, inertness, and relatively low immunogenicity. Moreover, PEG has been shown to support the viability of chondrocytes along with the deposition of new ECM by the cells (Bryant and Anseth, 2002). PCL is an FDA-approved synthetic scaffold, which is widely used in TE because of its tunable mechanical strength. PCL can be prepared as an electrospun nanofibrous scaffold or porous scaffold depending on the desired application (Ding et al., 2014; Panadero et al., 2016). Ceramic scaffolds including tricalcium phosphate and hydroxyapatite are biocompatible and can promote osteoinduction but they are poorly absorbed and brittle (Ghassemi et al., 2018; Roddy et al., 2018). Synthetic polymers such as PLA are resorbable but have limited osteo-inductive capacity (Roddy et al., 2018).
4.1.2 Natural Scaffolds
Scaffolds derived from natural sources used in chondral repair are generally prepared from collagen, hyaluronic acid, chitosan, silk, or alginate. The bioactivity, degradability, and biocompatibility of natural polymers make them desirable materials for TE (Jeuken et al., 2016; Li et al., 2018). Natural scaffolds are often prepared in the form of highly hydrated viscoelastic matrices known as hydrogels, which possess tunable swelling and mechanical properties based on the degree and type of cross-linking. These materials inherently provide the binding sites for cells, thus allowing cell-ECM interactions similar to native tissues (Catoira et al., 2019; Mantha et al., 2019).
Chitosan and alginate are among the natural polysaccharides that are widely used in cartilage repair and have displayed promising results (Xu et al., 2008; Yao et al., 2016; Ewa-Choy et al., 2017; Henrionnet et al., 2017; Merlin Rajesh Lal et al., 2017; Ruvinov et al., 2018; Huang et al., 2019). Based on our literature survey, chitosan has mainly been analyzed in combination for osteochondral defects and not alone. Alginate is derived from seaweeds, is both biocompatible and biodegradable, and is composed of beta-1-glucuronic acid and alpha-D-mannuronic acid. Several studies have reported that it supports chondrogenic proliferation, morphology, and the synthesis of type II collagen and proteoglycans (Homicz et al., 2003; Caron et al., 2012; Angelozzi et al., 2017; Aurich et al., 2018). Furthermore, stromal/stem cells derived from adipose, bone marrow, Wharton’s jelly, and dental pulp undergo chondrogenic differentiation when seeded on alginate scaffolds (Huang et al., 2015; Reppel et al., 2015; Ewa-Choy et al., 2017; Mata et al., 2017; Baba et al., 2018). The incorporation of mammalian compounds such as collagen into alginate has been shown to enhance the attachment and proliferation of cells (Bian et al., 2011; Lee and Mooney, 2012; Ganesh et al., 2013). Chitosan, prepared by the deacetylation of chitin, is another natural biomaterial used for OC regeneration. Because of its in vivo biocompatibility, degradability, and anti-bacterial properties, chitosan is widely used in TE (Cheung et al., 2015; Varun et al., 2017; Huang et al., 2019). It supports the chondrogenic differentiation of MSCs, chondrocytes, proliferation, and cartilaginous ECM deposition under in vitro and in vivo conditions (Griffon et al., 2006; Elder et al., 2013; Faikrua et al., 2013; Sheehy et al., 2015; Huang et al., 2019; Scalzone et al., 2019). However, chitosan presents poor mechanical properties requiring combination with other stiffer materials or the addition of crosslinking agents to optimize its activity in OC tissue engineering (Muzzarelli et al., 2015; De Mori et al., 2019; Kusmono and Abdurrahim, 2019; Scalzone et al., 2019; Islam et al., 2020; Jiang et al., 2022; Liu et al., 2022).
Gelatin methacryloyl (GelMa) hydrogels (semi-synthetic scaffold) have been widely investigated in TE because of their tunable physical and biological properties. The presence of cell attachment sites and MMP responsiveness allows stromal/stem cells to migrate and proliferate within GelMa in a manner similar to the native ECM (Yue et al., 2015). GelMa has been used in the regeneration of cardiovascular-like tissues (Chen et al., 2013), bone (Heo et al., 2014; Qiao et al., 2020), liver (Wu et al., 2020), and skin (Zhao et al., 2016).
Collagen is the most abundant ECM protein present in the animal kingdom. It provides high biocompatibility for cells and displays load-bearing capability due to its fibrous structure (Meyer, 2019). Consequently, collagen is used in many biological and medical products such as dental repair and wound healing (Costa et al., 2017). Nevertheless, collagen displays limitations in its physical properties such a lower mechanical strength than synthetic materials and its susceptibility to enzymatic degradation. Collagen modification by crosslinking to enhance stiffness and reduce degradability partially addresses these limitations and enhances its application for TE (Liu et al., 2019).
5 Adipose-Derived Stromal/Stem Cell-Scaffold Composites for Osteochondral Defect Regeneration
The ASCs’ mesenchymal differentiation capability is supported by a substantial body of literature demonstrating the efficacy and potential utility of ASCs for OC regeneration and defect repair (Bunnell, 2021). Since the direct application of ASCs to bone is hampered because of the stiffness and dry nature of the tissue, ASCs seeded on a scaffold presents a more practical mode of cell transplantation. Numerous biomaterials have been analyzed for their compatibility with ASCs and their subsequent capability to regenerate subchondral bone as composite biomaterials (Im et al., 2012; Lim et al., 2013; Boyer et al., 2020). Because of the favorable properties of hydrogels (biocompatibility, permeability, high water content, and tunable mechanical properties), they have been the most frequently studied biomaterial for OC defect regeneration (Guan et al., 2017).
5.1 Adipose-Derived Stromal/Stem Cell–Chitosan Scaffold
Chitosan has the ability to support cartilaginous ECM deposition and chondrocyte attachment which makes it a favorable scaffold for OC regeneration. The in vitro and in vivo attachment and proliferation of ASCs, BMSC, and chondrocyte have been reported by several studies (Griffon et al., 2006; Elder et al., 2013; Faikrua et al., 2013; Sheehy et al., 2015; Huang et al., 2019; Scalzone et al., 2019). ASCs seeded on a composite hydrogel of silanized-hydroxypropyl methylcellulose (Si-HPMC) mixed with silanized chitosan were found to remain viable both in vitro and after subcutaneous implantation in nude mice. Moreover, this ASC-seeded hydrogel exhibited significant regeneration in a canine model of OC defect (Boyer et al., 2020).
In another study, the investigators analyzed ASC-seeded chitosan/gelatin hydrogel and cancellous bone composite scaffold for the regeneration of bone and cartilage. The ASCs were induced to chondrocytes and osteoblasts before implantation in the hydrogel and their subsequent viability, proliferation, and ECM deposition were analyzed. The cells displayed significant proliferation in the composite scaffold and deposited cell-specific ECM which was confirmed by staining and scanning electron microscopy (SEM) (Song et al., 2016). These findings are significant since they can host cells and allow remodeling of its microenvironment, a highly desirable trait for a scaffold.
Articular cartilage relies on its biomechanical and biochemical interplay with the subchondral bone to maintain tissue health (Lories and Luyten, 2011). Therefore, for the regeneration of OC defects, a scaffold that supports the regeneration of cartilage and bone simultaneously is ideal, which is a feature often found missing in general scaffolds. To mitigate this challenge, a poly(l-glutamic acid/chitosan and hydroxyapatite-graft-poly (l-glutamic acid)) scaffold was prepared for the regeneration of both hyaline-like cartilage and underlying subchondral bone. The scaffold was found to support the chondrogenic differentiation of ASCs and spheroid formation. In addition, a chondrogenic ASC spheroid-seeded scaffold successfully regenerated hyaline cartilage along with the underlying subchondral bone in a rabbit-based model of OC defects (Zhang et al., 2016).
5.2 Adipose-Derived Stromal/Stem Cell–Calcium Alginate Scaffold
Alginate-based scaffolds with growth factors are widely used for bone (Kolambkar et al., 2011) and cartilage repair (Mierisch et al., 2002). Stem cells are facilitated to differentiate into bone and cartilage cells by using BMP4. It also facilitates the cells to deposit collagen type I and collagen type II and the in vivo regeneration of bone and cartilage (Kuroda et al., 2006; Zhang et al., 2008). Calcium alginate (CaAlg) hydrogels fabricated with BMP4-transduced ASCs were found to reconstruct the subchondral bone along with the formation of smooth and flat cartilage surfaces. Because of the upregulation of cytoplasmic BMP4, the secretion of collagen I, collagen II, and alkaline phosphatase was also increased. The deposition of these materials enhanced the differentiation of bone and cartilage cells (Chen et al., 2019).
5.3 Adipose-Derived Stromal/Stem Cell–Collagen Scaffold
Type 1 collagen is the most abundant component of the ECM (Yoneno et al., 2005) and a useful material for tissue engineering (Dong and Lv, 2016). A study has reported that the use of scaffolds along with growth factors such as BMP2 enhances the proliferation, attachment, and differentiation of ASCs (Lin et al., 2013). ASCs seeded on a collagen type 1 scaffold have displayed enhanced differentiation to OC lineage compared to two-dimensional (2D) cultures. The differentiation further increased when an ASC-collagen composite was cultured with media supplemented with platelet-rich plasma (PRP) and insulin. The expression of beta-1/beta-3 integrin was increased while it was found that the differentiation was independent of the mammalian target of rapamycin (mTOR) signaling and insulin-like growth factor 1 receptor (IGF-1R) (Scioli et al., 2016).
5.4 Adipose-Derived Stromal/Stem Cell–Cartilage-Based Scaffolds
Cartilage-based scaffolds have advantages over other scaffold materials since they contain the native cartilaginous ECM materials crucial for proliferation, attachment, and providing cues for the differentiation of cells. ASCs seeded on cartilage ECM-derived particles (CEDPs) differentiate into chondrocytes without the addition of any exogenous growth factor and with higher efficiency than the 2D-cultured ASCs. ASC-laden CEDPs displayed robust regeneration of rabbit hyaline cartilage, which was limited to fibrous tissue repair only when CEDP was used in the absence of ASCs (Yin et al., 2018).
A biodegradable porous sponge cartilage (BPSC) scaffold has been developed for the regeneration of hyaline-like cartilage. The BPSC scaffold was supplemented with ASCs or its secretome. The BPSC scaffolds fabricated with ASCs were found to regenerate the OC defect more efficiently than the scaffold fabricated with secretome alone (Widhiyanto et al., 2020). Kim et al. (2021) prepared PCL nanofibrils filled with decellularized cartilage ECM which were used along with ASCs. The ASCs seeded on the nanofibrils displayed chondrogenic differentiation without using any exogenous factors and cytokines, which was confirmed by the upregulation of cartilage marker genes. The ASC–nanofibril composite formed a clay-like structure that compactly filled the OC defect in rats and regenerated the cartilage and underlying bone.
Like articular cartilage, auricular cartilage comprises a GAG-collagen type II matrix with limited cell distribution. In contrast to articular cartilage, it has an elastic fiber network which surrounds the cells enabling the uniform distribution of cells. When enzymes are applied to remove the cells, it forms a hollow channel network which enables uniform distribution of cells on repopulation of the decellularized matrix. Bovine auricular cartilage scaffolds were repopulated with bovine and human chondrocytes in monoculture or co-culture with ASCs, which were found to increase cartilage regeneration with a high cell repopulation efficiency (Nürnberger et al., 2019).
5.5 Adipose-Derived Stromal/Stem Cell–Oligo (Polyethylene Glycol) Fumarate Scaffold
The oligo (PEG) fumarate (OPF) scaffold has been well characterized in previous studies (Dadsetan et al., 2012). The OPF scaffold has previously shown positive regenerative results in porcine OC defect models, where the scaffold was seeded with BMSCs before implantation (Lim et al., 2013). The OPF scaffold when seeded with autologous and human-derived ASCs exhibited a good quality of regeneration in a pig-based OC defect model compared to an unseeded scaffold. It was also found that type II collagen was expressed at higher levels in the ASCs seeded on an OPF scaffold, while the formation of mature subchondral bone was also observed, characterized by type I collagen expression (De Girolamo et al., 2015).
5.6 Adipose-Derived Stromal/Stem Cell–Polycaprolactone Scaffold
Because of its biocompatibility, flexibility, and biodegradability, polycaprolactone (PCL) is one of the most commonly used polyesters in medical applications. PCL-based scaffolds display slow degradation and maintenance of long-term structural integrity during in vitro culture. In addition, MSCs derived from the umbilical cord, bone marrow, and adipose tissue differentiate and synthesize bone and cartilaginous matrix when seeded on a PCL scaffold (Kim et al., 2010; Xue et al., 2017). Im and Lee (2010) investigated the comparison of a PCL scaffold seeded with ASCs and immobilized growth factors TGF-β2 and BMP-7 for the regeneration of OC defects in rabbits. Interestingly, the use of growth factors significantly improved the macroscopic scores of the OC defect but failed to improve the histological scores. It was also noted that the ASC-seeded scaffolds had an uncertain effect on the cartilage repair outcome, which was not found to be significantly better than the scaffold alone. The comparable performance of the scaffold alone could have been due to the infiltration of bone marrow stem cells into the scaffold from the surrounding tissue (Im and Lee, 2010).
5.7 Adipose-Derived Stromal/Stem Cell-3D Bioprinted Scaffolds
Since it is essential to recapitulate the complex fiber arrangement and pore size for optimal TE, 3D bioprinting has emerged as a useful tool to attain these requirements. A recent study has reported the development of 3D-printed scaffolds that are capable of hosting ASCs and regenerating site-specific OC defects. To drive site-specific ASC osteogenesis and chondrogenesis, a scaffold was fabricated using 3D-bioplotting of biodegradable PCL with TCP, or decellularized bovine cartilage ECM (dECM). The PCL-TCP scaffolds were found to be osteo-inductive whereas the PCL-dECM scaffolds favored chondrogenic differentiation of ASCs. Furthermore, a triphasic full-thickness OC scaffold was developed containing layers of PCL, PCL-TCP, and PCL-dECM to mimic the OC unit. ASCs were seeded on the triphasic scaffold and the histochemical analysis of the scaffold after 28 days of culture revealed that the scaffold was positive for calcium and GAGs in the PCL-TCP and PCL-dECM segments, respectively (Mellor et al., 2020). The in vivo evaluation of the same triphasic scaffold in the pig OC defect model revealed that lesions filled with scaffolds along with ASCs showed improved therapeutic effects compared to open lesions. However, although the scaffold facilitated subchondral bone regeneration, cartilage regeneration was found to be limited (Nordberg et al., 2021).
6 Adipose-Derived Stromal/Stem Cell–Scaffold Interaction
The interaction of the cells and scaffold is a critical factor in TE that helps the cells grow in natural biomimetic conditions (Chen et al., 2018). For OC regeneration, the scaffold should support the formation of both bone and cartilage throughout the regenerative process and should ideally resorb over time. Several studies have shown that the scaffolds not only allow attachment and proliferation of ASCs but also determine the cellular fate. In addition, the ASCs also remodel their environment while differentiating along different lineages. Scaffold composition, stiffness, porosity, and other physicochemical features have been found to influence the proliferation and differentiation of ASCs. Supplementation of scaffolds with ingredients that support osteogenic or chondrogenic differentiation of ASCs has been found to enhance their OC regenerative potential. A comparison of PLA scaffolds with or without the addition of tricalcium phosphate (TCP) showed that ASCs preferentially underwent osteogenic differentiation in the presence of TCP, whereas higher chondrogenesis was observed in the absence of TCP (Mellor et al., 2015). Biomimetic modifications of the PCL scaffold by the addition of collagen and fibronectin have been found to result in enhanced proliferation, colonization, and osteogenic differentiation of ASCs (Declercq et al., 2014). It has also been observed that ASCs, when seeded on PGA scaffolds, deposit a higher amount of GAGs and total collagen than in pellet culture (Mahmoudifar and Doran, 2010).
The microarchitecture of scaffolds too has the potential to influence cellular activity. ASCs seeded on a PCL scaffold having a modified nanowire surface have been shown to acquire an elongated morphology as opposed to non-elongated morphology when seeded on a smooth surface PCL scaffold. Moreover, ASCs cultured on the nanowire surface PCL scaffold displayed lower chondrogenesis than the smooth PCL scaffold (Trujillo and Popat, 2014). The pore size of the scaffold significantly influences the interaction between cells and the scaffold. The penetration and migration of the cells in scaffolds can be limited when the pore size is too small, whereas if the pore size is too wide, it can hamper cell adhesion (Murphy et al., 2010; Oh et al., 2010). Therefore, optimizing the pore size is critical for the fabrication of an efficient scaffold (Trujillo and Popat, 2014). A study conducted to determine the impact of pore size on ASC function revealed that pores ranging between 370–400 µm in size provide an optimal chondrogenic environment (Gómez et al., 2016).
The remodeling of scaffolds by differentiating ASCs has been reported by several recent studies. Mohiuddin et al. (2019b) showed that osteogenically differentiated ASCs seeded on decellularized adipose matrix remodeled the scaffold by mineral deposition and MMP-mediated rearrangement of ECM fibers. Similarly, other reports suggest that osteogenically and chondrogenically differentiating ASCs deposit calcium phosphates and GAGs, respectively, in the scaffolds (Im et al., 2012; Kim et al., 2021).
7 Present Challenges and Future Directions in Adipose-Derived Stromal/Stem Cell-Scaffold-Based Osteochondral Regeneration
7.1 Recreating the Bone Cartilage Interface
The calcified layer of cartilage present above the subchondral plate acts as a barrier between the bone and cartilage (Findlay and Kuliwaba, 2016). Some studies have suggested that the communication between the calcified cartilage and subchondral bone occurs through numerous vascular canals (Clark and Huber, 1990). The holes present in the subchondral plate open into the bone marrow space that connects the OC unit (Duncan et al., 1987). Moreover, upon culturing bovine osteochondral explants, it was found that the chondrocytes died more rapidly after 7 days in the absence of subchondral bone, whereas when cultured in the presence of subchondral bone, the chondrocytes remained viable. This phenomenon was potentially a result of the survival factor(s) provided by the bone as observed between the bone and cartilage under physiological conditions (Amin et al., 2009).
Recapitulating the interface between the bone and cartilage is one of the most significant challenges in OC TE. Earlier, some biphasic grafts were developed to support the growth of both bone and cartilage as separate tissues (Chu et al., 1995; Gao et al., 2001; Malda et al., 2005; Getgood et al., 2012). However, they do not optimally mimic the native OC interphase, since they partially support bone and cartilage regeneration in separated scaffold layers (Schek et al., 2004; Keeney and Pandit, 2009). Based on the structure of OC tissue, there is a need for matrices that support tissue regeneration and present an interactive bone–cartilage interface (Athanasiou et al., 2009). To cope with this problem, gradient scaffolds are being developed that support osteogenesis and chondrogenesis, while providing a native tissue-like transition between cartilage and bone layers. These scaffolds have been evaluated both in vitro and in vivo (Sherwood et al., 2002; Martin et al., 2007; Wang et al., 2009; Mohan et al., 2011; Declercq et al., 2014; Yousefi et al., 2015). A gradient scaffold comprising poly(D, L-lactic-co-glycolic acid), containing TGFβ1 and BMP2, with or without hydroxyapatite, showed a significant extent of OC regeneration in rabbits (Mohan et al., 2011). Several other gradient scaffolds in combination with various cell types have shown promising results for the treatment of OC defects (Du et al., 2017; Gao et al., 2018).
To better understand the intra-articular tissue cross-talk and the etiology of OC defects, Li et al. (2022) have developed an MSC-derived miniature joint system containing OC tissue, fibrous tissue, and adipose tissue. Such systems will not only enhance the understanding of the OC unit but can potentially be adapted to engineer patient-specific OC units for transplantation in the future.
7.2 Availability of Clinical-Grade Adipose-Derived Stromal/Stem Cells
ASCs are a good candidate for OC defect regeneration because of their easy availability, rich source, and biocompatibility with various types of scaffolds. However, some limitations must be addressed for the successful clinical translation of ASC-scaffold composites. The use of autologous ASCs is considered safe for therapeutic applications as they are not expected to elicit an immune response. However, obtaining a sufficient amount of tissue to isolate a clinically efficacious number of cells is a major challenge (Zhang et al., 2015). To overcome this issue, ASCs can be culture-expanded to increase the cell number (Kharbanda et al., 2014). Another potential solution is the use of allogeneic stem cells. However, the availability of adipose tissue is dependent on surgical procedures, which limits the availability of tissue from healthy donors. Another limitation in using allogeneic ASCs is the variability at the cellular and molecular levels as a consequence of donor sex, age, BMI, and tissue depot (Shu et al., 2012; Bodle et al., 2014; Ock et al., 2016; Abbo et al., 2017).
To produce clinical-grade ASCs, good manufacturing practice (GMP)-based manufacturing facilities need to be established where ASCs obtained from healthy donors are isolated and culture-expanded under aseptic conditions. The ASCs isolated from multiple donors can then be pooled to minimize donor-based variability in the final batch of cells to be used for therapeutic purposes (Kuçi et al., 2016) (Ankrum et al., 2014; Patrikoski et al., 2014). A major impediment in the large-scale culture of clinical-grade cells is the availability of a non-xenogeneic source of growth factors. Animal-derived serum is presently the gold standard for experimental cell culture; however, for clinical translation, human serum and platelet lysate are being analyzed.
The optimal concentration of ASCs to be seeded on scaffolds for OC regeneration is another area that needs increased focus and harmonization. A wide range of ASC doses has been tested for OC regeneration thus far, details of which are shown in Table 1. For the clinical translation of ASC-scaffold composites, it is imperative that future studies are conducted to determine the dose-specific effect of these composites on OC regeneration.
TABLE 1.
In vitro and preclinical application of ASC-scaffold composites.
| S. No. | ASC dose | Source of ASCs | Scaffold type | Model | Reference |
|---|---|---|---|---|---|
| 1 | 2 × 106/ml | Human | Silylated chitosan and cellulose hydrogel | Canine OC defect in dogs | Boyer et al. (2020) |
| 2 | 0.02 × 106 cells/spheroid | Human | 3D-printed osteochondral interface using osteogenic and chondrogenic spheroid | In vitro study | Ayan et al. (2020) |
| 3 | 0.5 × 106 cell/40 ml | Rabbit | Porous poly (ε-caprolactone) (PCL) scaffold with different pore sizes | OC defect in femur of rabbit | Im et al. (2012) |
| 4 | 0.2 × 106 cells/350 μl | Human | 3D collagen gel scaffold | In vitro study | Scioli et al. (2016) |
| 5 | 1 × 107 cells/ml | Rabbit | Coacervate-embedded composite hydrogels | Femoral trochlear osteochondral defect in rabbit | Cho et al. (2020) |
| 6 | 0.25 × 106/cm2 (when 0% tricalcium phosphate used) and 0.02 × 106/cm2 (when 20% TCP used) | Human | Stacked polylactic acid nanofibrous scaffolds | In vitro study | Mellor et al. (2015) |
| 7 | 1 × 107 cells/ml | Human | Cancellous bone/hydrogel (chitosan/gelatin) hybrid scaffold | In vitro study | Song et al. (2016) |
| 8 | 2 × 106 cells | Rabbit | Cartilage extracellular matrix-derived particles (CEDPs) and cartilage slice-based scaffold | Rabbit femoral trochlear osteochondral defect | Yin et al. (2018) |
| 9 | 0.4 × 106 cells/30 ul | Human immortalized ASCs (Evercyte, Cat# CHT-001-0005) | Photo-crosslinked gelatin methacryloyl (gelMA) scaffold | In vitro study | Hölzl et al. (2021) |
| 10 | 0.256 × 106 per microspheroids | Human immortalized ASCs (Evercyte, Cat# CHT-001-0005) | Gelatin-based hydrogels | In vitro study | Zigon-Branc et al. (2019) |
| 11 | 0.25 × 106 cells for the chondrogenic layer and 0.5 × 106 cells for the osteogenic layer | Rabbit | Trilayered silk fibroin scaffolds | In vitro study | Ding et al. (2014) |
| 12 | 0.25 × 106 cells/scaffold | Human | Multiphasic 3D-bioplotted scaffolds | In vitro study | Mellor et al. (2020) |
| 13 | 2 × 106 cells/scaffold | Rabbit | Biodegradable porous sponge cartilage scaffold | Full thickness femoral defect in rabbits | Widhiyanto et al. (2020) |
| 14 | 0.5 × 106 cells/ml (in vivo) 5 × 103 cells/ml–0.5 × 106 cells/ml (in vitro) | Not specified | Protein-reactive nanofibrils scaffold | Articular cartilage defect | Kim et al. (2021) |
| 15 | 50 ul of 50 × 106 cells/ml | Rabbit | Poly(L-glutamic acid)-based scaffold | Articular osteochondral defect in rabbits | Zhang et al. (2016) |
| 16 | 3 × 106 cells | Minipig human | Oligo (polyethylene glycol) fumarate (OPF) hydrogel | OC defect in minipigs | De Girolamo et al. (2015) |
| 17 | 0.5 × 106 cells | Rabbit | Immobilized porous polycaprolactone scaffold | Distal femur OC defect in rabbits | Im and Lee, (2010) |
| 18 | 0.25 × 106 co-cultured cells with human articular chondrocytes | Human immortalized ASCs (Evercyte, Cat# CHT-001-0005) | AuriScaff (auricular cartilage scaffold) | OC plug model in mice | Nürnberger et al. (2019) |
| 19 | 0.075 × 106 and 0.15 × 106 cells | Pig ASCs | Multiphasic 3D-bioplotted scaffold | OC defects in minipigs | Nordberg et al. (2021) |
7.3 Elucidation of Adipose-Derived Stromal/Stem Cell Paracrine Activity
Immunomodulatory and anti-inflammatory capacities are the most clinically relevant properties of ASCs. Several studies have confirmed that ASCs carry out these activities via paracrine signaling via the upregulation of anti-inflammatory cytokines while suppressing pro-inflammatory molecules (Melief et al., 2013; Carrade Holt et al., 2014; Bowles et al., 2017). ASCs have been found to suppress the proliferation and migration of activated inflammatory cells in case of arthritis, thus preventing bone and cartilage degradation (Ter Huurne et al., 2012; Manferdini et al., 2017; Ueyama et al., 2020). Although the role of ASC paracrine activity in tissue generation via modulation of immune and inflammatory response is well established, the present literature relating to the use of ASC-scaffold composites for OC regeneration has placed very little emphasis on the elucidation of these mechanisms. Further studies are mandatory to establish the understanding of molecular mechanisms involved in the regeneration of OC defects by these composites. Moreover, it will be interesting to evaluate if ASCs display enhanced or diminished paracrine activity when implanted alone or in combination with scaffolds.
8 Clinical Trials
Based on our search using www.clinicaltrials.gov, twelve clinical trials have been initiated to test the OC regenerative capability of the scaffolds. These studies involve the use of scaffolds and/or different types of cells. Details of the clinical trials are provided in Table 2 (www.clinicaltrials.gov).
TABLE 2.
Clinical trials for osteochondral defects.
| S. No. | Title | Condition | Scaffold used | No. of participants | Study start date | Expected end date | Country | Status |
|---|---|---|---|---|---|---|---|---|
| 1 | Repair of Articular Osteochondral Defect | Osteochondritis dissecans | Biphasic osteochondral composite | 10 | March 2009 | December 2011 | Taiwan | Unknown status |
| 2 | A Study to Evaluate the Efficacy of BioCartilage® Micronized Cartilage Matrix in Microfracture Treatment of Osteochondral Defects | Osteochondral defect | BioCartilage® micronized cartilage matrix | 15 | January 2019 | November 2023 | Canada | Recruiting |
| 3 | Follow-up Study Evaluating the Long-Term Outcome of ChondroMimetic in the Treatment of Osteochondral Defects in the Knee | Osteochondral defect | ChondroMimetic | 15 | May 2017 | February 2018 | Hungary | Completed |
| 4 | A Prospective, Post-Marketing Registry on the Use of ChondroMimetic for the Repair of Osteochondral Defects | Osteochondral defects | Chondromimetic | 8 | September 2010 | April 2013 | Hungary | Terminated |
| 5 | A Study to Evaluate the Safety of Augment™ Bone Graft | Defect of articular cartilage | Augment Bone Graft | 1 | July 2011 | August 2012 | Canada | Completed |
| 6 | Transplantation of Bone Marrow Stem Cells Stimulated by Protein Scaffolds to Heal Defects in Articular Cartilage of the Knee | Osteoarthritis|knee Osteoarthritis|osteochondritis | Transplantation of bone marrow stem cells | 50 | July 2010 | December 2014 | France | Unknown status |
| 7 | The Effectiveness of Adding Allogenic Stem Cells After Traditional Treatment of Osteochondral Lesions of the Talus (OLT) | Osteochondral fracture of talus | Allogenic stromal mesenchymal cells derived from the umbilical cord | platelet-poor plasma Scaffold | 70 | 15 January 2019 | December 2024 | Chile | Recruiting |
| 8 | One-Step Bone Marrow Mononuclear Cell Transplantation in Talar Osteochondral lesions | Osteochondritis | Procedure: bone marrow cells transplantation on collagen scaffold | 140 | April 2013 | April 2018 | Italy | Unknown |
| 9 | Study for the Treatment of Knee Chondral and Osteochondral Lesions | Knee chondral lesion knee osteochondral lesion |
Procedure: Marrow stimulation—drilling or microfractures device: MaioRegen surgery | 145 | January 2011 | February 2016 | Europe and South Africa | Completed |
| 10 | Triphasic Osteochondral Scaffold for the Treatment of the OCD of the Knee: Observational Study | Osteochondritis dissecans knee | Triphasic Scaffold | 30 | 1 April 2022 | April 2029 | Italy | Recruiting |
| 11 | Evaluation of an Acellular Osteochondral Graft for Cartilage Lesions Pilot Trial (EAGLE Pilot) | Articular cartilage injury | Device: Kensey Nash Corp. Cartilage repair device | 2 | June 2010 | May 2014 | United States | Terminated |
| 12 | Biphasic Cartilage Repair Implant (BiCRI) IDE Clinical Trial-Taiwan | Chondral or osteochondral lesion of medial femoral condyle chondral or osteochondral lesion of lateral femoral condyle chondral or osteochondral lesion of trochlea | Device: Biphasic cartilage repair implant | 92 | October 2011 | August 2019 | Taiwan | Completed |
9 Conclusion
Despite the recent advancements in OC tissue engineering, there is still a need to optimize natural and synthetic biomaterials that can repair OC defects. The scaffolds when implanted along with the ASCs show a higher regenerative efficacy than the use of ASCs or scaffolds alone. However, presently, there is an advantage of using scaffolds alone as a cell-free system as it mitigates the regulatory complications related to the application of ASCs. Most of the current studies to treat OC defects are in the experimental phase. There is a need to carry out clinical trials translating promising results from pre-clinical animal models. To date, few clinical trials have been conducted thus far to analyze the safety and efficacy of ASC-scaffold composites. With the completion of present ongoing clinical trials and others to follow, there will be greater clarity about the future course of action in terms of the optimal scaffold design and cell seeding strategy. An ideal OC scaffold should support both osteogenesis and chondrogenesis, which while being interrelated, are distinct phenomena. A promising future strategy appears to be the development of layered scaffolds which contain osteogenic and chondrogenic compartments as reported by Mellor et al. (2020) and Nordberg et al. (2021). Such scaffolds recapitulate the physiological OC structure and are therefore exciting prospects for the regeneration of OC defects and in vitro OC engineering. Moreover, the availability of clinical-grade allogeneic ASCs is also required to advance the field, since autologous ASCs are generally not available in sufficient numbers and quality to efficiently regenerate physiological defects.
Overall, while the development of ASC-scaffold composites for OC regeneration is in its early stages of research, the available results are promising but will require further validation in human patients under protocols approved and evaluated by internationally recognized regulatory authorities.
Author Contributions
OM and JG conceived the idea and reviewed/edited the manuscript. GR surveyed the literature and authored the manuscript, and TF reviewed/edited the manuscript.
Conflict of Interest
TF and JG are co-founders, co-owners, and employees of Obatala Sciences Inc., a for-profit biotechnology company focused on adipose tissue-derived cells and extracellular matrix products for regenerative medical research and clinical translation.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- Abbo O., Taurand M., Monsarrat P., Raymond I., Arnaud E., De Barros S., et al. (2017). Comparison between Pediatric and Adult Adipose Mesenchymal Stromal Cells. Cytotherapy 19, 395–407. 10.1016/J.JCYT.2016.11.012 PubMed Abstract | 10.1016/J.JCYT.2016.11.012 | Google Scholar [DOI] [PubMed] [Google Scholar]
- Afessa B., Peters S. (2006). Major Complications Following Hematopoietic Stem Cell Transplantation. Semin. Respir. Crit. Care Med. 27, 297–309. 10.1055/S-2006-945530 PubMed Abstract | 10.1055/S-2006-945530 | Google Scholar [DOI] [PubMed] [Google Scholar]
- Amin A. K., Huntley J. S., Simpson A. H. R. W., Hall A. C. (2009). Chondrocyte Survival in Articular Cartilage. J. Bone Jt. Surg. Br. volume 91-B, 691–699. 10.1302/0301-620X.91B5.21544 PubMed Abstract | 10.1302/0301-620X.91B5.21544 | Google Scholar [DOI] [PubMed] [Google Scholar]
- Angelozzi M., Penolazzi L., Mazzitelli S., Lambertini E., Lolli A., Piva R., et al. (2017). Dedifferentiated Chondrocytes in Composite Microfibers as Tool for Cartilage Repair. Front. Bioeng. Biotechnol. 5, 35. 10.3389/FBIOE.2017.00035/BIBTEX PubMed Abstract | 10.3389/FBIOE.2017.00035/BIBTEX | Google Scholar [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ankrum J. A., Ong J. F., Karp J. M. (2014). Mesenchymal Stem Cells: Immune Evasive, Not Immune Privileged. Nat. Biotechnol. 32, 252–260. 10.1038/nbt.2816 PubMed Abstract | 10.1038/nbt.2816 | Google Scholar [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argentati C., Morena F., Bazzucchi M., Armentano I., Emiliani C., Martino S. (2018). Adipose Stem Cell Translational Applications: from Bench-To-Bedside. Ijms 19, 3475. 10.3390/ijms19113475 PubMed Abstract | 10.3390/ijms19113475 | Google Scholar [DOI] [PMC free article] [PubMed] [Google Scholar]
- Athanasiou K. A., Darling E. M., Hu J. C. (2009). Articular Cartilage Tissue Engineering. Synth. Lect. Tissue Eng. 1, 1–182. Available at: https://scholar.google.com.pk/scholar?hl=en&as_sdt=0%2C5&q=Articular+cartilage+tissue+engineering+Synth.+Lect.+Tissue+Eng.+1+1–182&btnG= (Accessed April 19, 2022). 10.2200/s00212ed1v01y200910tis003 | Google Scholar [DOI] [Google Scholar]
- Aurich M., Hofmann G. O., Best N., Rolauffs B. (2018). Induced Redifferentiation of Human Chondrocytes from Articular Cartilage Lesion in Alginate Bead Culture after Monolayer Dedifferentiation: An Alternative Cell Source for Cell-Based Therapies? Tissue Eng. Part A 24, 275–286. 10.1089/TEN.TEA.2016.0505 PubMed Abstract | 10.1089/TEN.TEA.2016.0505 | Google Scholar [DOI] [PubMed] [Google Scholar]
- Ayan B., Wu Y., Karuppagounder V., Kamal F., Ozbolat I. T. (2020). Aspiration-assisted Bioprinting of the Osteochondral Interface. Sci. Rep. 10. 10.1038/s41598-020-69960-6 PubMed Abstract | 10.1038/s41598-020-69960-6 | Google Scholar [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baba R., Onodera T., Matsuoka M., Hontani K., Joutoku Z., Matsubara S., et al. (2018). Bone Marrow Stimulation Technique Augmented by an Ultrapurified Alginate Gel Enhances Cartilage Repair in a Canine Model. Am. J. Sports Med. 46, 1970–1979. 10.1177/0363546518770436 PubMed Abstract | 10.1177/0363546518770436 | Google Scholar [DOI] [PubMed] [Google Scholar]
- Bacakova L., Zarubova J., Travnickova M., Musilkova J., Pajorova J., Slepicka P., et al. (2018). Stem Cells: Their Source, Potency and Use in Regenerative Therapies with Focus on Adipose-Derived Stem Cells - a Review. Biotechnol. Adv. 36, 1111–1126. 10.1016/j.biotechadv.2018.03.011 PubMed Abstract | 10.1016/j.biotechadv.2018.03.011 | Google Scholar [DOI] [PubMed] [Google Scholar]
- Barbatelli G., Murano I., Madsen L., Hao Q., Jimenez M., Kristiansen K., et al. (2010). The Emergence of Cold-Induced Brown Adipocytes in Mouse White Fat Depots Is Determined Predominantly by White to Brown Adipocyte Transdifferentiation. Am. J. Physiology-Endocrinology Metabolism 298, E1244–E1253. 10.1152/AJPENDO.00600.2009 10.1152/AJPENDO.00600.2009 | Google Scholar [DOI] [PubMed] [Google Scholar]
- Bian L., Zhai D. Y., Tous E., Rai R., Mauck R. L., Burdick J. A. (2011). Enhanced MSC Chondrogenesis Following Delivery of TGF-Β3 from Alginate Microspheres within Hyaluronic Acid Hydrogels In Vitro and In Vivo . Biomaterials 32, 6425–6434. 10.1016/J.BIOMATERIALS.2011.05.033 PubMed Abstract | 10.1016/J.BIOMATERIALS.2011.05.033 | Google Scholar [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodle J. C., Teeter S. D., Hluck B. H., Hardin J. W., Bernacki S. H., Loboa E. G. (2014). Age-related Effects on the Potency of Human Adipose-Derived Stem Cells: Creation and Evaluation of Superlots and Implications for Musculoskeletal Tissue Engineering Applications. Tissue Eng. Part C. Methods 20, 972–983. 10.1089/TEN.TEC.2013.0683/ASSET/IMAGES/LARGE/FIGURE8.JPEG PubMed Abstract | 10.1089/TEN.TEC.2013.0683/ASSET/IMAGES/LARGE/FIGURE8.JPEG | Google Scholar [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourin P., Bunnell B. A., Casteilla L., Dominici M., Katz A. J., March K. L., et al. (2013). Stromal Cells from the Adipose Tissue-Derived Stromal Vascular Fraction and Culture Expanded Adipose Tissue-Derived Stromal/stem Cells: a Joint Statement of the International Federation for Adipose Therapeutics and Science (IFATS) and the International Society for Cellular Therapy (ISCT)Stromal Cells from the Adipose Tissue-Derived Stromal Vascular Fraction and Culture Expanded Adipose Tissue-Derived Stromal/stem Cells: a Joint Statement of. Cytotherapy 15, 641–648. 10.1016/j.jcyt.2013.02.006 PubMed Abstract | 10.1016/j.jcyt.2013.02.006 | Google Scholar [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowles A. C., Wise R. M., Gerstein B. Y., Thomas R. C., Ogelman R., Febbo I., et al. (2017). Immunomodulatory Effects of Adipose Stromal Vascular Fraction Cells Promote Alternative Activation Macrophages to Repair Tissue Damage. Stem Cells 35, 2198–2207. 10.1002/STEM.2689 PubMed Abstract | 10.1002/STEM.2689 | Google Scholar [DOI] [PubMed] [Google Scholar]
- Boyer C., Réthoré G., Weiss P., d’Arros C., Lesoeur J., Vinatier C., et al. (2020). A Self-Setting Hydrogel of Silylated Chitosan and Cellulose for the Repair of Osteochondral Defects: From In Vitro Characterization to Preclinical Evaluation in Dogs. Front. Bioeng. Biotechnol. 8. 10.3389/fbioe.2020.00023 PubMed Abstract | 10.3389/fbioe.2020.00023 | Google Scholar [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan M. A., Renaud A., Guilloton F., Mebarki M., Trichet V., Sensebé L., et al. (2017). Inferior In Vivo Osteogenesis and Superior Angiogeneis of Human Adipose-Derived Stem Cells Compared with Bone Marrow-Derived Stem Cells Cultured in Xeno-free Conditions. Stem Cells Transl. Med. 6, 2160–2172. 10.1002/SCTM.17-0133 PubMed Abstract | 10.1002/SCTM.17-0133 | Google Scholar [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant S. J., Anseth K. S. (2002). Hydrogel Properties Influence ECM Production by Chondrocytes Photoencapsulated in Poly(ethylene Glycol) Hydrogels. J. Biomed. Mat. Res. 59, 63–72. 10.1002/JBM.1217 PubMed Abstract | 10.1002/JBM.1217 | Google Scholar [DOI] [PubMed] [Google Scholar]
- Buckwalter J. A., Anderson D. D., Brown T. D., Tochigi Y., Martin J. A. (2013). The Roles of Mechanical Stresses in the Pathogenesis of Osteoarthritis. Cartilage 4, 286–294. 10.1177/1947603513495889 PubMed Abstract | 10.1177/1947603513495889 | Google Scholar [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunnell B. A. (2021). Adipose Tissue-Derived Mesenchymal Stem Cells. Cells 10, 3433. 10.3390/cells10123433 PubMed Abstract | 10.3390/cells10123433 | Google Scholar [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caron M. M. J., Emans P. J., Coolsen M. M. E., Voss L., Surtel D. A. M., Cremers A., et al. (2012). Redifferentiation of Dedifferentiated Human Articular Chondrocytes: Comparison of 2D and 3D Cultures. Osteoarthr. Cartil. 20, 1170–1178. 10.1016/J.JOCA.2012.06.016 10.1016/J.JOCA.2012.06.016 | Google Scholar [DOI] [PubMed] [Google Scholar]
- Carrade Holt D. D., Wood J. A., Granick J. L., Walker N. J., Clark K. C., Borjesson D. L. (2014). Equine Mesenchymal Stem Cells Inhibit T Cell Proliferation through Different Mechanisms Depending on Tissue Source. Stem Cells Dev. 23, 1258–1265. 10.1089/SCD.2013.0537/ASSET/IMAGES/LARGE/FIGURE4.JPEG PubMed Abstract | 10.1089/SCD.2013.0537/ASSET/IMAGES/LARGE/FIGURE4.JPEG | Google Scholar [DOI] [PubMed] [Google Scholar]
- Catoira M. C., Fusaro L., Di Francesco D., Ramella M., Boccafoschi F. (2019). Overview of Natural Hydrogels for Regenerative Medicine Applications. J. Mater Sci. Mater Med. 30, 1–10. 10.1007/S10856-019-6318-7 10.1007/S10856-019-6318-7 | Google Scholar [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang S. H., Mori D., Kobayashi H., Mori Y., Nakamoto H., Okada K., et al. (2019). Excessive Mechanical Loading Promotes Osteoarthritis through the Gremlin-1-NF-Κb Pathway. Nat. Commun. 10. 10.1038/S41467-019-09491-5 10.1038/S41467-019-09491-5 | Google Scholar [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H., Sun J., Wang Z., Zhou Y., Lou Z., Chen B., et al. (2018). Magnetic Cell-Scaffold Interface Constructed by Superparamagnetic IONP Enhanced Osteogenesis of Adipose-Derived Stem Cells. ACS Appl. Mat. Interfaces 10, 44279–44289. 10.1021/ACSAMI.8B17427 PubMed Abstract | 10.1021/ACSAMI.8B17427 | Google Scholar [DOI] [PubMed] [Google Scholar]
- Chen L., Shi Y., Zhang X., Hu X., Shao Z., Dai L., et al. (2019). CaAlg Hydrogel Containing Bone Morphogenetic Protein 4-enhanced Adipose-Derived Stem Cells Combined with Osteochondral Mosaicplasty Facilitated the Repair of Large Osteochondral Defects. Knee Surg. Sports Traumatol. Arthrosc. 27, 3668–3678. 10.1007/s00167-019-05418-1 PubMed Abstract | 10.1007/s00167-019-05418-1 | Google Scholar [DOI] [PubMed] [Google Scholar]
- Chen M. B., Srigunapalan S., Wheeler A. R., Simmons C. A. (2013). A 3D Microfluidic Platform Incorporating Methacrylated Gelatin Hydrogels to Study Physiological Cardiovascular Cell-Cell Interactions. Lab. Chip 13, 2591–2598. 10.1039/C3LC00051F PubMed Abstract | 10.1039/C3LC00051F | Google Scholar [DOI] [PubMed] [Google Scholar]
- Cheung R., Ng T., Wong J., Chan W. (2015). Chitosan: An Update on Potential Biomedical and Pharmaceutical Applications. Mar. Drugs 13, 5156–5186. 10.3390/MD13085156 PubMed Abstract | 10.3390/MD13085156 | Google Scholar [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chimutengwende-Gordon M., Donaldson J., Bentley G. (2020). Current Solutions for the Treatment of Chronic Articular Cartilage Defects in the Knee. EFORT Open Rev. 5, 156–163. 10.1302/2058-5241.5.190031 PubMed Abstract | 10.1302/2058-5241.5.190031 | Google Scholar [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho H., Kim J., Kim S., Jung Y. C., Wang Y., Kang B.-J., et al. (2020). Dual Delivery of Stem Cells and Insulin-like Growth Factor-1 in Coacervate-Embedded Composite Hydrogels for Enhanced Cartilage Regeneration in Osteochondral Defects. J. Control. Release 327, 284–295. 10.1016/j.jconrel.2020.08.002 PubMed Abstract | 10.1016/j.jconrel.2020.08.002 | Google Scholar [DOI] [PubMed] [Google Scholar]
- Chu C. R., Coutts R. D., Yoshioka M., Harwood F. L., Monosov A. Z., Amiel D. (1995). Articular Cartilage Repair Using Allogeneic Perichondrocyteseeded Biodegradable Porous Polylactic Acid (PLA): A Tissue-Engineering Study. J. Biomed. Mat. Res. 29, 1147–1154. 10.1002/JBM.820290915 10.1002/JBM.820290915 | Google Scholar [DOI] [PubMed] [Google Scholar]
- Clark J., Huber J. (1990). The Structure of the Human Subchondral Plate. J. Bone Jt. Surg. Br. volume 72-B, 866–873. 10.1302/0301-620X.72B5.2211774 PubMed Abstract | 10.1302/0301-620X.72B5.2211774 | Google Scholar [DOI] [PubMed] [Google Scholar]
- clinicaltrials.gov (2021). clinicaltrials.gov. Available at: www.clinicaltrials.gov (Accessed February 15, 2022). Google Scholar
- Clouet J., Vinatier C., Merceron C., Pot-vaucel M., Maugars Y., Weiss P., et al. (2009). From Osteoarthritis Treatments to Future Regenerative Therapies for Cartilage. Drug Discov. Today 14, 913–925. 10.1016/J.DRUDIS.2009.07.012 PubMed Abstract | 10.1016/J.DRUDIS.2009.07.012 | Google Scholar [DOI] [PubMed] [Google Scholar]
- Costa A., Naranjo J. D., Londono R., Badylak S. F. (2017). Biologic Scaffolds. Cold Spring Harb. Perspect. Med. 7, a025676. 10.1101/CSHPERSPECT.A025676 PubMed Abstract | 10.1101/CSHPERSPECT.A025676 | Google Scholar [DOI] [PMC free article] [PubMed] [Google Scholar]
- Critchley S., Sheehy E. J., Cunniffe G., Diaz-Payno P., Carroll S. F., Jeon O., et al. (2020). 3D Printing of Fibre-Reinforced Cartilaginous Templates for the Regeneration of Osteochondral Defects. Acta Biomater. 113, 130–143. 10.1016/J.ACTBIO.2020.05.040 PubMed Abstract | 10.1016/J.ACTBIO.2020.05.040 | Google Scholar [DOI] [PubMed] [Google Scholar]
- Cvetković V. J., Takić Miladinov D., Stojanović S. (2018). Genotoxicity and Mutagenicity Testing of Biomaterials. Biomater. Clin. Pract., 501–527. 10.1007/978-3-319-68025-5_18 10.1007/978-3-319-68025-5_18 | Google Scholar [DOI] [Google Scholar]
- Dadsetan M., Giuliani M., Wanivenhaus F., Brett Runge M., Charlesworth J. E., Yaszemski M. J. (2012). Incorporation of Phosphate Group Modulates Bone Cell Attachment and Differentiation on Oligo(polyethylene Glycol) Fumarate Hydrogel. Acta Biomater. 8, 1430–1439. 10.1016/J.ACTBIO.2011.12.031 PubMed Abstract | 10.1016/J.ACTBIO.2011.12.031 | Google Scholar [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai Y., Shen T., Ma L., Wang D., Gao C. (2018). Regeneration of Osteochondral Defects In Vivo by a Cell‐free Cylindrical Poly(lactide‐co‐glycolide) Scaffold with a Radially Oriented Microstructure. J. Tissue Eng. Regen. Med. 12, e1647–e1661. 10.1002/TERM.2592 PubMed Abstract | 10.1002/TERM.2592 | Google Scholar [DOI] [PubMed] [Google Scholar]
- Davis S., Roldo M., Blunn G., Tozzi G., Roncada T. (2021). Influence of the Mechanical Environment on the Regeneration of Osteochondral Defects. Front. Bioeng. Biotechnol. 9, 10. 10.3389/FBIOE.2021.603408/FULL 10.3389/FBIOE.2021.603408/FULL | Google Scholar [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Girolamo L., Niada S., Arrigoni E., Di Giancamillo A., Domeneghini C., Dadsetan M., et al. (2015). Repair of Osteochondral Defects in the Minipig Model by OPF Hydrogel Loaded with Adipose-Derived Mesenchymal Stem Cells. Regen. Med. 10, 135–151. 10.2217/rme.14.77 PubMed Abstract | 10.2217/rme.14.77 | Google Scholar [DOI] [PubMed] [Google Scholar]
- De Mori A., Hafidh M., Mele N., Yusuf R., Cerri G., Gavini E., et al. (2019). Sustained Release from Injectable Composite Gels Loaded with Silver Nanowires Designed to Combat Bacterial Resistance in Bone Regeneration Applications. Pharmaceutics 11, 116. 10.3390/PHARMACEUTICS11030116 10.3390/PHARMACEUTICS11030116 | Google Scholar [DOI] [PMC free article] [PubMed] [Google Scholar]
- Declercq H. A., Desmet T., Dubruel P., Cornelissen M. J. (2014). The Role of Scaffold Architecture and Composition on the Bone Formation by Adipose-Derived Stem Cells. Tissue Eng. Part A 20, 434–444. 10.1089/TEN.TEA.2013.0179 PubMed Abstract | 10.1089/TEN.TEA.2013.0179 | Google Scholar [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding X., Zhu M., Xu B., Zhang J., Zhao Y., Ji S., et al. (2014). Integrated Trilayered Silk Fibroin Scaffold for Osteochondral Differentiation of Adipose-Derived Stem Cells. ACS Appl. Mat. Interfaces 6, 16696–16705. 10.1021/am5036708 10.1021/am5036708 | Google Scholar [DOI] [PubMed] [Google Scholar]
- Dong C., Lv Y. (2016). Application of Collagen Scaffold in Tissue Engineering: Recent Advances and New Perspectives. Polymers 8, 42. 10.3390/POLYM8020042 PubMed Abstract | 10.3390/POLYM8020042 | Google Scholar [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Y., Liu H., Yang Q., Wang S., Wang J., Ma J., et al. (2017). Selective Laser Sintering Scaffold with Hierarchical Architecture and Gradient Composition for Osteochondral Repair in Rabbits. Biomaterials 137, 37–48. 10.1016/J.BIOMATERIALS.2017.05.021 PubMed Abstract | 10.1016/J.BIOMATERIALS.2017.05.021 | Google Scholar [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan H., Jundt J., Riddle J. M., Pitchford W., Christopherson T. (1987). The Tibial Subchondral Plate. A Scanning Electron Microscopic Study. J. Bone & Jt. Surg. 69, 1212–1220. 10.2106/00004623-198769080-00015 10.2106/00004623-198769080-00015 | Google Scholar [DOI] [PubMed] [Google Scholar]
- Elder S., Gottipati A., Zelenka H., Bumgardner J. (2013). Attachment, Proliferation, and Chondroinduction of Mesenchymal Stem Cells on Porous Chitosan-Calcium Phosphate Scaffolds. Toorthj 7, 275–281. 10.2174/1874325001307010275 10.2174/1874325001307010275 | Google Scholar [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estes B. T., Diekman B. O., Gimble J. M., Guilak F. (2010). Isolation of Adipose-Derived Stem Cells and Their Induction to a Chondrogenic Phenotype. Nat. Protoc. 5, 1294–1311. 10.1038/NPROT.2010.81 PubMed Abstract | 10.1038/NPROT.2010.81 | Google Scholar [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewa-Choy Y. W., Pingguan-Murphy B., Abdul-Ghani N. A., Jahendran J., Chua K. H. (2017). Effect of Alginate Concentration on Chondrogenesis of Co-cultured Human Adipose-Derived Stem Cells and Nasal Chondrocytes: a Biological Study. Biomater. Res. 21, 1–11. 10.1186/S40824-017-0105-7 PubMed Abstract | 10.1186/S40824-017-0105-7 | Google Scholar [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faikrua A., Wittaya-Areekul S., Oonkhanond B., Viyoch J. (2013). In Vivo chondrocyte and Transforming Growth Factor-Β1 Delivery Using the Thermosensitive Chitosan/starch/β-Glycerol Phosphate Hydrogel. J. Biomater. Appl. 28, 175–186. 10.1177/0885328212441847 PubMed Abstract | 10.1177/0885328212441847 | Google Scholar [DOI] [PubMed] [Google Scholar]
- Fan H., Tao H., Wu Y., Hu Y., Yan Y., Luo Z. (2010). TGF-β3 Immobilized PLGA-Gelatin/chondroitin Sulfate/hyaluronic Acid Hybrid Scaffold for Cartilage Regeneration. J. Biomed. Mat. Res. 95A, 982–992. 10.1002/JBM.A.32899 10.1002/JBM.A.32899 | Google Scholar [DOI] [PubMed] [Google Scholar]
- Fang Y., Zhang T., Song Y., Sun W. (2020). Assessment of Various Crosslinking Agents on Collagen/chitosan Scaffolds for Myocardial Tissue Engineering. Biomed. Mat. 15, 045003. 10.1088/1748-605X/AB452D 10.1088/1748-605X/AB452D | Google Scholar [DOI] [PubMed] [Google Scholar]
- Farré-Guasch E., Bravenboer N., Helder M., Schulten E., ten Bruggenkate C., Klein-Nulend J. (2018). Blood Vessel Formation and Bone Regeneration Potential of the Stromal Vascular Fraction Seeded on a Calcium Phosphate Scaffold in the Human Maxillary Sinus Floor Elevation Model. Materials 11, 161. 10.3390/MA11010161 10.3390/MA11010161 | Google Scholar [DOI] [PMC free article] [PubMed] [Google Scholar]
- Findlay D. M., Kuliwaba J. S. (2016). Bone-cartilage Crosstalk: a Conversation for Understanding Osteoarthritis. Bone Res. 4. 10.1038/BONERES.2016.28 10.1038/BONERES.2016.28 | Google Scholar [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser J. K., Wulur I., Alfonso Z., Zhu M., Wheeler E. S. (2007). Differences in Stem and Progenitor Cell Yield in Different Subcutaneous Adipose Tissue Depots. Cytotherapy 9, 459–467. 10.1080/14653240701358460 PubMed Abstract | 10.1080/14653240701358460 | Google Scholar [DOI] [PubMed] [Google Scholar]
- Ganesh N., Hanna C., Nair S. V., Nair L. S. (2013). Enzymatically Cross-Linked Alginic-Hyaluronic Acid Composite Hydrogels as Cell Delivery Vehicles. Int. J. Biol. Macromol. 55, 289–294. 10.1016/J.IJBIOMAC.2012.12.045 PubMed Abstract | 10.1016/J.IJBIOMAC.2012.12.045 | Google Scholar [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao F., Xu Z., Liang Q., Liu B., Li H., Wu Y., et al. (2018). Direct 3D Printing of High Strength Biohybrid Gradient Hydrogel Scaffolds for Efficient Repair of Osteochondral Defect. Adv. Funct. Mat. 28, 1706644. 10.1002/ADFM.201706644 10.1002/ADFM.201706644 | Google Scholar [DOI] [Google Scholar]
- Gao J., Dennis J. E., Solchaga L. A., Awadallah A. S., Goldberg V. M., Caplan A. I. (2001). Tissue-engineered Fabrication of an Osteochondral Composite Graft Using Rat Bone Marrow-Derived Mesenchymal Stem Cells. Tissue Eng. 7, 363–371. 10.1089/10763270152436427 PubMed Abstract | 10.1089/10763270152436427 | Google Scholar [DOI] [PubMed] [Google Scholar]
- Getgood A. M. J., Kew S. J., Brooks R., Aberman H., Simon T., Lynn A. K., et al. (2012). Evaluation of Early-Stage Osteochondral Defect Repair Using a Biphasic Scaffold Based on a Collagen-Glycosaminoglycan Biopolymer in a Caprine Model. Knee 19, 422–430. 10.1016/J.KNEE.2011.03.011 PubMed Abstract | 10.1016/J.KNEE.2011.03.011 | Google Scholar [DOI] [PubMed] [Google Scholar]
- Ghassemi T., Shahroodi A., Ebrahimzadeh M. H., Mousavian A. (2018). Current Concepts in Scaffolding for Bone Tissue Engineering. Arch. bone Jt. Surg. 6, 90–99. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/pmc5867363/(Accessed February 27, 2022). PubMed Abstract | Google Scholar [PMC free article] [PubMed] [Google Scholar]
- Girolamo L., Sartori M. F., Albisetti W. T., Brini A. T. (20072007). Osteogenic Differentiation of Human Adipose-Derived Stem Cells: Comparison of Two Different Inductive mediaOsteogenic Differentiation of Human Adipose‐derived Stem Cells: Comparison of Two Different Inductive Media. J. Tissue Eng. Regen. Med. 1, 154–157. 10.1002/term.12 10.1002/term.12 | Google Scholar [DOI] [PubMed] [Google Scholar]
- Gomes M. E., Reis R. L. (2004). Tissue Engineering: Key Elements and Some Trends. Macromol. Biosci. 4, 737–742. 10.1002/MABI.200400094 PubMed Abstract | 10.1002/MABI.200400094 | Google Scholar [DOI] [PubMed] [Google Scholar]
- Gómez S., Vlad M. D., López J., Fernández E. (2016). Design and Properties of 3D Scaffolds for Bone Tissue Engineering. Acta Biomater. 42, 341–350. 10.1016/J.ACTBIO.2016.06.032 PubMed Abstract | 10.1016/J.ACTBIO.2016.06.032 | Google Scholar [DOI] [PubMed] [Google Scholar]
- Grafe I., Alexander S., Peterson J. R., Snider T. N., Levi B., Lee B., et al. (2018). TGF-β Family Signaling in Mesenchymal Differentiation. Cold Spring Harb. Perspect. Biol. 10, a022202. 10.1101/CSHPERSPECT.A022202 PubMed Abstract | 10.1101/CSHPERSPECT.A022202 | Google Scholar [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffon D., Sedighi M., Schaeffer D., Eurell J., Johnson A. (2006). Chitosan Scaffolds: Interconnective Pore Size and Cartilage Engineering. Acta Biomater. 2, 313–320. 10.1016/J.ACTBIO.2005.12.007 PubMed Abstract | 10.1016/J.ACTBIO.2005.12.007 | Google Scholar [DOI] [PubMed] [Google Scholar]
- Grigore M. E. (2017). Biomaterials for Cartilage Tissue Engineering. J. tissue Sci. Eng. 08, 192. 10.4172/2157-7552.1000192 10.4172/2157-7552.1000192 | Google Scholar [DOI] [Google Scholar]
- Grimm N. L., Weiss J. M., Kessler J. I., Aoki S. K. (2014). Osteochondritis Dissecans of the Knee. Clin. Sports Med. 33, 181–188. 10.1016/J.CSM.2013.11.006 PubMed Abstract | 10.1016/J.CSM.2013.11.006 | Google Scholar [DOI] [PubMed] [Google Scholar]
- Guan X., Avci-Adali M., Alarçin E., Cheng H., Kashaf S. S., Li Y., et al. (2017). Development of Hydrogels for Regenerative Engineering. Biotechnol. J. 12, 1600394. 10.1002/BIOT.201600394 10.1002/BIOT.201600394 | Google Scholar [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henrionnet C., Liang G., Roeder E., Dossot M., Wang H., Magdalou J., et al. (2017). Hypoxia for Mesenchymal Stem Cell Expansion and Differentiation: The Best Way for Enhancing TGFß-Induced Chondrogenesis and Preventing Calcifications in Alginate Beads. Tissue Eng. Part A 23, 913–922. 10.1089/TEN.TEA.2016.0426 PubMed Abstract | 10.1089/TEN.TEA.2016.0426 | Google Scholar [DOI] [PubMed] [Google Scholar]
- Heo D. N., Ko W.-K., Bae M. S., Lee J. B., Lee D.-W., Byun W., et al. (2014). Enhanced Bone Regeneration with a Gold Nanoparticle-Hydrogel Complex. J. Mat. Chem. B 2, 1584–1593. 10.1039/x0xx00000x 10.1039/x0xx00000x | Google Scholar [DOI] [PubMed] [Google Scholar]
- Himms-Hagen J., Melnyk A., Zingaretti M. C., Ceresi E., Barbatelli G., Cinti S. (2000). Multilocular Fat Cells in WAT of CL-316243-Treated Rats Derive Directly from White Adipocytes. Am. J. Physiology-Cell Physiology 279, C670–C681. 10.1152/AJPCELL.2000.279.3.C670 PubMed Abstract | 10.1152/AJPCELL.2000.279.3.C670 | Google Scholar [DOI] [PubMed] [Google Scholar]
- Hiraki Y., Shukunami C., Iyama K., Mizuta H. (2001). Differentiation of Chondrogenic Precursor Cells during the Regeneration of Articular Cartilage. Osteoarthr. Cartil. 9, S102–S108. 10.1016/S1063-4584(01)94436-X 10.1016/S1063-4584(01)94436-X | Google Scholar [DOI] [PubMed] [Google Scholar]
- Hölzl K., Fürsatz M., Göcerler H., Schädl B., Žigon‐Branc S., Markovic M., et al. (2021). Gelatin Methacryloyl as Environment for Chondrocytes and Cell Delivery to Superficial Cartilage Defects. J. Tissue Eng. Regen. Med. 16, 207–222. 10.1002/term.3273 PubMed Abstract | 10.1002/term.3273 | Google Scholar [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homicz M. R., Chia S. H., Schumacher B. L., Masuda K., Thonar E. J., Sah R. L., et al. (2003). Human Septal Chondrocyte Redifferentiation in Alginate, Polyglycolic Acid Scaffold, and Monolayer Culture. Laryngoscope 113, 25–32. 10.1097/00005537-200301000-00005 PubMed Abstract | 10.1097/00005537-200301000-00005 | Google Scholar [DOI] [PubMed] [Google Scholar]
- Hu W., Chen Y., Dou C., Dong S. (2020). Microenvironment in Subchondral Bone: Predominant Regulator for the Treatment of Osteoarthritis. Ann. Rheum. Dis. 80, 413–422. 10.1136/ANNRHEUMDIS-2020-218089 PubMed Abstract | 10.1136/ANNRHEUMDIS-2020-218089 | Google Scholar [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y., Seitz D., König F., Müller P. E., Jansson V., Klar R. M. (2019). Induction of Articular Chondrogenesis by Chitosan/Hyaluronic-Acid-Based Biomimetic Matrices Using Human Adipose-Derived Stem Cells. Ijms 20, 4487. 10.3390/IJMS20184487 PubMed Abstract | 10.3390/IJMS20184487 | Google Scholar [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Z., Nooeaid P., Kohl B., Roether J. A., Schubert D. W., Meier C., et al. (2015). Chondrogenesis of Human Bone Marrow Mesenchymal Stromal Cells in Highly Porous Alginate-Foams Supplemented with Chondroitin Sulfate. Mater. Sci. Eng. C 50, 160–172. 10.1016/J.MSEC.2015.01.082 10.1016/J.MSEC.2015.01.082 | Google Scholar [DOI] [PubMed] [Google Scholar]
- Hunter D. J., Schofield D., Callander E. (2014). The Individual and Socioeconomic Impact of Osteoarthritis. Nat. Rev. Rheumatol. 10, 437–441. 10.1038/NRRHEUM.2014.44 PubMed Abstract | 10.1038/NRRHEUM.2014.44 | Google Scholar [DOI] [PubMed] [Google Scholar]
- Im G.-I., Ko J.-Y., Lee J. H. (2012). Chondrogenesis of Adipose Stem Cells in a Porous Polymer Scaffold: Influence of the Pore Size. Cell Transpl. 21, 2397–2405. 10.3727/096368912X638865 PubMed Abstract | 10.3727/096368912X638865 | Google Scholar [DOI] [PubMed] [Google Scholar]
- Im G.-I., Lee J. H. (2009). Repair of Osteochondral Defects with Adipose Stem Cells and a Dual Growth Factor-Releasing Scaffold in Rabbits. J. Biomed. Mat. Res. 9999B, NA. 10.1002/jbm.b.31552 10.1002/jbm.b.31552 | Google Scholar [DOI] [PubMed] [Google Scholar]
- Islam M. M., Shahruzzaman M., Biswas S., Nurus Sakib M., Rashid T. U. (2020). Chitosan Based Bioactive Materials in Tissue Engineering Applications-A Review. Bioact. Mater. 5, 164–183. 10.1016/J.BIOACTMAT.2020.01.012 PubMed Abstract | 10.1016/J.BIOACTMAT.2020.01.012 | Google Scholar [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeuken R., Roth A., Peters R., van Donkelaar C., Thies J., van Rhijn L., et al. (2016). Polymers in Cartilage Defect Repair of the Knee: Current Status and Future Prospects. Polymers 8, 219. 10.3390/POLYM8060219 PubMed Abstract | 10.3390/POLYM8060219 | Google Scholar [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang S., Qiao C., Wang X., Li Z., Yang G. (2022). Structure and Properties of Chitosan/sodium Dodecyl Sulfate Composite Films. RSC Adv. 12, 3969–3978. 10.1039/D1RA08218C PubMed Abstract | 10.1039/D1RA08218C | Google Scholar [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajimura S., Spiegelman B. M., Seale P. (2015). Brown and Beige Fat: Physiological Roles beyond Heat Generation. Cell Metab. 22, 546–559. 10.1016/J.CMET.2015.09.007 PubMed Abstract | 10.1016/J.CMET.2015.09.007 | Google Scholar [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karageorgiou V., Kaplan D. (2005). Porosity of 3D Biomaterial Scaffolds and Osteogenesis. Biomaterials 26, 5474–5491. 10.1016/J.BIOMATERIALS.2005.02.002 PubMed Abstract | 10.1016/J.BIOMATERIALS.2005.02.002 | Google Scholar [DOI] [PubMed] [Google Scholar]
- Keane T. J., Badylak S. F. (2014). Biomaterials for Tissue Engineering Applications. Seminars Pediatr. Surg. 23, 112–118. 10.1053/J.SEMPEDSURG.2014.06.010 10.1053/J.SEMPEDSURG.2014.06.010 | Google Scholar [DOI] [PubMed] [Google Scholar]
- Keeney M., Pandit A. (2009). The Osteochondral Junction and its Repair via Bi-phasic Tissue Engineering Scaffolds. Tissue Eng. Part B Rev. 15, 55–73. 10.1089/TEN.TEB.2008.0388 PubMed Abstract | 10.1089/TEN.TEB.2008.0388 | Google Scholar [DOI] [PubMed] [Google Scholar]
- Khan S., Ul-Islam M., Ikram M., Islam S. U., Ullah M. W., Israr M., et al. (2018). Preparation and Structural Characterization of Surface Modified Microporous Bacterial Cellulose Scaffolds: A Potential Material for Skin Regeneration Applications In Vitro and In Vivo . Int. J. Biol. Macromol. 117, 1200–1210. 10.1016/J.IJBIOMAC.2018.06.044 PubMed Abstract | 10.1016/J.IJBIOMAC.2018.06.044 | Google Scholar [DOI] [PubMed] [Google Scholar]
- Kharbanda S., Smith A. R., Hutchinson S. K., McKenna D. H., Ball J. B., Lamb L. S., et al. (2014). Unrelated Donor Allogeneic Hematopoietic Stem Cell Transplantation for Patients with Hemoglobinopathies Using a Reduced-Intensity Conditioning Regimen and Third-Party Mesenchymal Stromal Cells. Biol. Blood Marrow Transplant. 20, 581–586. 10.1016/J.BBMT.2013.12.564 PubMed Abstract | 10.1016/J.BBMT.2013.12.564 | Google Scholar [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H.-J., Lee J.-H., Im G.-I. (2009). Chondrogenesis Using Mesenchymal Stem Cells and PCL Scaffolds. J. Biomed. Mat. Res. 9999A, NA. 10.1002/JBM.A.32414 10.1002/JBM.A.32414 | Google Scholar [DOI] [PubMed] [Google Scholar]
- Kim H. S., Mandakhbayar N., Kim H.-W., Leong K. W., Yoo H. S. (2021). Protein-reactive Nanofibrils Decorated with Cartilage-Derived Decellularized Extracellular Matrix for Osteochondral Defects. Biomaterials 269, 120214. 10.1016/j.biomaterials.2020.120214 PubMed Abstract | 10.1016/j.biomaterials.2020.120214 | Google Scholar [DOI] [PubMed] [Google Scholar]
- Kolambkar Y. M., Dupont K. M., Boerckel J. D., Huebsch N., Mooney D. J., Hutmacher D. W., et al. (2011). An Alginate-Based Hybrid System for Growth Factor Delivery in the Functional Repair of Large Bone Defects. Biomaterials 32, 65–74. 10.1016/J.BIOMATERIALS.2010.08.074 PubMed Abstract | 10.1016/J.BIOMATERIALS.2010.08.074 | Google Scholar [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krasnokutsky S., Belitskaya-Lévy I., Bencardino J., Samuels J., Attur M., Regatte R., et al. (2011). Quantitative Magnetic Resonance Imaging Evidence of Synovial Proliferation Is Associated with Radiographic Severity of Knee Osteoarthritis. Arthritis & Rheumatism 63, 2983–2991. 10.1002/ART.30471 10.1002/ART.30471 | Google Scholar [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuci Z., Bonig H., Kreyenberg H., Bunos M., Jauch A., Janssen J. W. G., et al. (2016). Mesenchymal Stromal Cells from Pooled Mononuclear Cells of Multiple Bone Marrow Donors as Rescue Therapy in Pediatric Severe Steroid-Refractory Graft-Versus-Host Disease: a Multicenter Survey. Haematologica 101, 985–994. 10.3324/HAEMATOL.2015.140368 PubMed Abstract | 10.3324/HAEMATOL.2015.140368 | Google Scholar [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudva A., Luyten F., Patterson J. (2018). In Vitro Screening of Molecularly Engineered Polyethylene Glycol Hydrogels for Cartilage Tissue Engineering Using Periosteum-Derived and ATDC5 Cells. Ijms 19, 3341. 10.3390/IJMS19113341 PubMed Abstract | 10.3390/IJMS19113341 | Google Scholar [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuroda R., Usas A., Kubo S., Corsi K., Peng H., Rose T., et al. (2006). Cartilage Repair Using Bone Morphogenetic Protein 4 and Muscle-Derived Stem Cells. Arthritis Rheum. 54, 433–442. 10.1002/ART.21632 PubMed Abstract | 10.1002/ART.21632 | Google Scholar [DOI] [PubMed] [Google Scholar]
- Kusmono, Abdurrahim I. (2019). Water Sorption, Antimicrobial Activity, and Thermal and Mechanical Properties of Chitosan/clay/glycerol Nanocomposite Films. Heliyon 5, e02342. 10.1016/J.HELIYON.2019.E02342 PubMed Abstract | 10.1016/J.HELIYON.2019.E02342 | Google Scholar [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuterbekov M., Machillot P., Lhuissier P., Picart C., Jonas A. M., Glinel K. (2018). Solvent-free Preparation of Porous Poly(l-Lactide) Microcarriers for Cell Culture. Acta Biomater. 75, 300–311. 10.1016/j.actbio.2018.06.009 PubMed Abstract | 10.1016/j.actbio.2018.06.009 | Google Scholar [DOI] [PubMed] [Google Scholar]
- Kuzmenko V., Karabulut E., Pernevik E., Enoksson P., Gatenholm P. (2018). Tailor-made Conductive Inks from Cellulose Nanofibrils for 3D Printing of Neural Guidelines. Carbohydr. Polym. 189, 22–30. 10.1016/J.CARBPOL.2018.01.097 PubMed Abstract | 10.1016/J.CARBPOL.2018.01.097 | Google Scholar [DOI] [PubMed] [Google Scholar]
- Langer R., Vacanti J. (2016). Advances in Tissue Engineering. J. Pediatr. Surg. 51, 8–12. 10.1016/J.JPEDSURG.2015.10.022 PubMed Abstract | 10.1016/J.JPEDSURG.2015.10.022 | Google Scholar [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanza R., Langer R., Vacanti J., Atala A. (2020). Principles of Tissue Engineering. 5th Edition. Available at: https://www.elsevier.com/books/principles-of-tissue-engineering/lanza/978-0-12-818422-6 (Accessed March 1, 2022). Google Scholar [Google Scholar]
- Lee E. J., Kasper F. K., Mikos A. G. (2014). Biomaterials for Tissue Engineering. Ann. Biomed. Eng. 42, 323–337. 10.1007/S10439-013-0859-6 PubMed Abstract | 10.1007/S10439-013-0859-6 | Google Scholar [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K. Y., Mooney D. J. (2012). Alginate: Properties and Biomedical Applications. Prog. Polym. Sci. 37, 106–126. 10.1016/J.PROGPOLYMSCI.2011.06.003 PubMed Abstract | 10.1016/J.PROGPOLYMSCI.2011.06.003 | Google Scholar [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L., Yu F., Zheng L., Wang R., Yan W., Wang Z., et al. (2019). Natural Hydrogels for Cartilage Regeneration: Modification, Preparation and Application. J. Orthop. Transl. 17, 26–41. 10.1016/J.JOT.2018.09.003 PubMed Abstract | 10.1016/J.JOT.2018.09.003 | Google Scholar [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z., Lin Z., Liu S., Yagi H., Zhang X., Yocum L., et al. (2022). Human Mesenchymal Stem Cell‐Derived Miniature Joint System for Disease Modeling and Drug Testing. Adv. Sci., 2105909. 10.1002/advs.202105909 10.1002/advs.202105909 | Google Scholar [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim C. T., Ren X., Afizah M. H., Tarigan-Panjaitan S., Yang Z., Wu Y., et al. (2013). Repair of Osteochondral Defects with Rehydrated Freeze-Dried Oligo[poly(ethylene Glycol) Fumarate] Hydrogels Seeded with Bone Marrow Mesenchymal Stem Cells in a Porcine Model. Tissue Eng. Part A 19, 1852–1861. 10.1089/TEN.TEA.2012.0621 PubMed Abstract | 10.1089/TEN.TEA.2012.0621 | Google Scholar [DOI] [PubMed] [Google Scholar]
- Lin C.-Y., Chang Y.-H., Li K.-C., Lu C.-H., Sung L.-Y., Yeh C.-L., et al. (2013). The Use of ASCs Engineered to Express BMP2 or TGF-Β3 within Scaffold Constructs to Promote Calvarial Bone Repair. Biomaterials 34, 9401–9412. 10.1016/J.BIOMATERIALS.2013.08.051 PubMed Abstract | 10.1016/J.BIOMATERIALS.2013.08.051 | Google Scholar [DOI] [PubMed] [Google Scholar]
- Lin Y., Chen X., Yan Z., Liu L., Tang W., Zheng X., et al. (2006). Multilineage Differentiation of Adipose-Derived Stromal Cells from GFP Transgenic Mice. Mol. Cell. Biochem. 285, 69–78. 10.1007/S11010-005-9056-8 PubMed Abstract | 10.1007/S11010-005-9056-8 | Google Scholar [DOI] [PubMed] [Google Scholar]
- Litowczenko J., Woźniak-Budych M. J., Staszak K., Wieszczycka K., Jurga S., Tylkowski B. (2021). Milestones and Current Achievements in Development of Multifunctional Bioscaffolds for Medical Application. Bioact. Mater. 6, 2412–2438. 10.1016/J.BIOACTMAT.2021.01.007 PubMed Abstract | 10.1016/J.BIOACTMAT.2021.01.007 | Google Scholar [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C.-H., Jiang H.-T., Wang C.-H. (2022). Fabrication and Characterization of a Toughened Spherical Chitosan Adsorbent Only through Physical Crosslinking Based on Mechanism of Chain Rearrangement. RSC Adv. 12, 9179–9185. 10.1039/D1RA09438F PubMed Abstract | 10.1039/D1RA09438F | Google Scholar [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu G., Zhang Y., Liu B., Sun J., Li W., Cui L., et al. (2013). Bone Regeneration in a Canine Cranial Model Using Allogeneic Adipose Derived Stem Cells and Coral Scaffold. Biomaterials 34, 2655–2664. 10.1016/j.biomaterials.2013.01.004 PubMed Abstract | 10.1016/j.biomaterials.2013.01.004 | Google Scholar [DOI] [PubMed] [Google Scholar]
- Liu X., Zheng C., Luo X., Wang X., Jiang H. (2019). Recent Advances of Collagen-Based Biomaterials: Multi-Hierarchical Structure, Modification and Biomedical Applications. Mater. Sci. Eng. C 99, 1509–1522. 10.1016/J.MSEC.2019.02.070 10.1016/J.MSEC.2019.02.070 | Google Scholar [DOI] [PubMed] [Google Scholar]
- Lo S.-C., Li K.-C., Chang Y.-H., Hsu M.-N., Sung L.-Y., Vu T. A., et al. (2017). Enhanced Critical-Size Calvarial Bone Healing by ASCs Engineered with Cre/loxP-Based Hybrid Baculovirus. Biomaterials 124, 1–11. 10.1016/J.BIOMATERIALS.2017.01.033 PubMed Abstract | 10.1016/J.BIOMATERIALS.2017.01.033 | Google Scholar [DOI] [PubMed] [Google Scholar]
- Lópiz-Morales Y., Abarrategi A., Abarrategi A., Ramos V., Moreno-Vicente C., López-Durán L., et al. (2010). In Vivo comparison of the Effects of RHBMP-2 and RHBMP-4 in Osteochondral Tissue Regeneration. eCM 20, 367–378. 10.22203/ECM.V020A30 PubMed Abstract | 10.22203/ECM.V020A30 | Google Scholar [DOI] [PubMed] [Google Scholar]
- Lories R. J., Luyten F. P. (2011). The Bone-Cartilage Unit in Osteoarthritis. Nat. Rev. Rheumatol. 7, 43–49. 10.1038/NRRHEUM.2010.197 PubMed Abstract | 10.1038/NRRHEUM.2010.197 | Google Scholar [DOI] [PubMed] [Google Scholar]
- Louwen F., Ritter A., Kreis N. N., Yuan J. (2018). Insight into the Development of Obesity: Functional Alterations of Adipose-Derived Mesenchymal Stem Cells. Obes. Rev. 19, 888–904. 10.1111/OBR.12679 PubMed Abstract | 10.1111/OBR.12679 | Google Scholar [DOI] [PubMed] [Google Scholar]
- Maged A., Abdelkhalek A. A., Mahmoud A. A., Salah S., Ammar M. M., Ghorab M. M. (2019). Mesenchymal Stem Cells Associated with Chitosan Scaffolds Loaded with Rosuvastatin to Improve Wound Healing. Eur. J. Pharm. Sci. 127, 185–198. 10.1016/J.EJPS.2018.11.002 PubMed Abstract | 10.1016/J.EJPS.2018.11.002 | Google Scholar [DOI] [PubMed] [Google Scholar]
- Mahmoudifar N., Doran P. M. (2010). Chondrogenic Differentiation of Human Adipose-Derived Stem Cells in Polyglycolic Acid Mesh Scaffolds under Dynamic Culture Conditions. Biomaterials 31, 3858–3867. 10.1016/J.BIOMATERIALS.2010.01.090 PubMed Abstract | 10.1016/J.BIOMATERIALS.2010.01.090 | Google Scholar [DOI] [PubMed] [Google Scholar]
- Malda J., Woodfield T. B. F., Van Der Vloodt F., Wilson C., Martens D. E., Tramper J., et al. (2005). The Effect of PEGT/PBT Scaffold Architecture on the Composition of Tissue Engineered Cartilage. Biomaterials 26, 63–72. 10.1016/J.BIOMATERIALS.2004.02.046 PubMed Abstract | 10.1016/J.BIOMATERIALS.2004.02.046 | Google Scholar [DOI] [PubMed] [Google Scholar]
- Manferdini C., Paolella F., Gabusi E., Gambari L., Piacentini A., Filardo G., et al. (2017). Adipose Stromal Cells Mediated Switching of the Pro-inflammatory Profile of M1-like Macrophages Is Facilitated by PGE2: In Vitro Evaluation. Osteoarthr. Cartil. 25, 1161–1171. 10.1016/J.JOCA.2017.01.011 10.1016/J.JOCA.2017.01.011 | Google Scholar [DOI] [PubMed] [Google Scholar]
- Mantha S., Pillai S., Khayambashi P., Upadhyay A., Zhang Y., Tao O., et al. (2019). Smart Hydrogels in Tissue Engineering and Regenerative Medicine. Materials 12, 3323. 10.3390/MA12203323 PubMed Abstract | 10.3390/MA12203323 | Google Scholar [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin I., Miot S., Barbero A., Jakob M., Wendt D. (2007). Osteochondral Tissue Engineering. J. Biomechanics 40, 750–765. 10.1016/J.JBIOMECH.2006.03.008 10.1016/J.JBIOMECH.2006.03.008 | Google Scholar [DOI] [PubMed] [Google Scholar]
- Mata M., Milian L., Oliver M., Zurriaga J., Sancho-Tello M., Llano J. J. M. d., et al. (2017). In Vivo Articular Cartilage Regeneration Using Human Dental Pulp Stem Cells Cultured in an Alginate Scaffold: A Preliminary Study. Stem Cells Int. 2017, 1–9. 10.1155/2017/8309256 PubMed Abstract | 10.1155/2017/8309256 | Google Scholar [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melief S. M., Zwaginga J. J., Fibbe W. E., Roelofs H. (2013). Adipose Tissue-Derived Multipotent Stromal Cells Have a Higher Immunomodulatory Capacity Than Their Bone Marrow-Derived Counterparts. Stem Cells Transl. Med. 2, 455–463. 10.5966/SCTM.2012-0184 PubMed Abstract | 10.5966/SCTM.2012-0184 | Google Scholar [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellor L. F., Mohiti-Asli M., Williams J., Kannan A., Dent M. R., Guilak F., et al. (2015). Extracellular Calcium Modulates Chondrogenic and Osteogenic Differentiation of Human Adipose-Derived Stem Cells: A Novel Approach for Osteochondral Tissue Engineering Using a Single Stem Cell Source. Tissue Eng. Part A 21, 2323–2333. 10.1089/ten.tea.2014.0572 PubMed Abstract | 10.1089/ten.tea.2014.0572 | Google Scholar [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellor L. F., Nordberg R. C., Huebner P., Mohiti‐Asli M., Taylor M. A., Efird W., et al. (20202017). Investigation of Multiphasic 3D‐bioplotted Scaffolds for Site‐specific Chondrogenic and Osteogenic Differentiation of Human Adipose‐derived Stem Cells for Osteochondral Tissue Engineering Applications. J. Biomed. Mater Res. 108, 2017–2030. 10.1002/JBM.B.34542 10.1002/JBM.B.34542 | Google Scholar [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merceron C., Vinatier C., Portron S., Masson M., Amiaud J., Guigand L., et al. (2010). Differential Effects of Hypoxia on Osteochondrogenic Potential of Human Adipose-Derived Stem Cells. Am. J. Physiology-Cell Physiology 298, C355–C364. 10.1152/AJPCELL.00398.2009 10.1152/AJPCELL.00398.2009 | Google Scholar [DOI] [PubMed] [Google Scholar]
- Merlin Rajesh Lal L. P., Suraishkumar G. K., Nair P. D. (2017). Chitosan-agarose Scaffolds Supports Chondrogenesis of Human Wharton's Jelly Mesenchymal Stem Cells. J. Biomed. Mat. Res. 105, 1845–1855. 10.1002/JBM.A.36054 10.1002/JBM.A.36054 | Google Scholar [DOI] [PubMed] [Google Scholar]
- Meyer M. (2019). Processing of Collagen Based Biomaterials and the Resulting Materials Properties. Biomed. Eng. Online 18, 24. 10.1186/S12938-019-0647-0 PubMed Abstract | 10.1186/S12938-019-0647-0 | Google Scholar [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mierisch C. M., Cohen S. B., Jordan L. C., Robertson P. G., Balian G., Diduch D. R. (2002). Transforming Growth Factor-β in Calcium Alginate Beads for the Treatment of Articular Cartilage Defects in the Rabbit. Arthrosc. J. Arthrosc. Relat. Surg. 18, 892–900. 10.1053/JARS.2002.36117 10.1053/JARS.2002.36117 | Google Scholar [DOI] [PubMed] [Google Scholar]
- Minteer D., Marra K. G., Rubin J. P. (2012). Adipose-Derived Mesenchymal Stem Cells: Biology and Potential Applications. Adv. Biochem. Eng. Biotechnol. 129, 59–71. 10.1007/10_2012_146 10.1007/10_2012_146 | Google Scholar [DOI] [PubMed] [Google Scholar]
- Mohan N., Dormer N. H., Caldwell K. L., Key V. H., Berkland C. J., Detamore M. S. (2011). Continuous Gradients of Material Composition and Growth Factors for Effective Regeneration of the Osteochondral Interface. Tissue Eng. Part A 17, 2845–2855. 10.1089/TEN.TEA.2011.0135 PubMed Abstract | 10.1089/TEN.TEA.2011.0135 | Google Scholar [DOI] [PubMed] [Google Scholar]
- Mohiuddin O. A., Campbell B., Poche J. N., Ma M., Rogers E., Gaupp D., et al. (2019a). Decellularized Adipose Tissue Hydrogel Promotes Bone Regeneration in Critical-Sized Mouse Femoral Defect Model. Front. Bioeng. Biotechnol. 7. 10.3389/FBIOE.2019.00211 PubMed Abstract | 10.3389/FBIOE.2019.00211 | Google Scholar [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohiuddin O. A., O’Donnell B. T., Poche J. N., Iftikhar R., Wise R. M., Motherwell J. M., et al. (2019b). Human Adipose-Derived Hydrogel Characterization Based on In Vitro ASC Biocompatibility and Differentiation. Stem Cells Int. 2019, 1–13. 10.1155/2019/9276398 PubMed Abstract | 10.1155/2019/9276398 | Google Scholar [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mumme M., Barbero A., Miot S., Wixmerten A., Feliciano S., Wolf F., et al. (2016). Nasal Chondrocyte-Based Engineered Autologous Cartilage Tissue for Repair of Articular Cartilage Defects: an Observational First-In-Human Trial. Lancet 388, 1985–1994. 10.1016/S0140-6736(16)31658-0 PubMed Abstract | 10.1016/S0140-6736(16)31658-0 | Google Scholar [DOI] [PubMed] [Google Scholar]
- Murphy C. M., Haugh M. G., O'Brien F. J. (2010). The Effect of Mean Pore Size on Cell Attachment, Proliferation and Migration in Collagen-Glycosaminoglycan Scaffolds for Bone Tissue Engineering. Biomaterials 31, 461–466. 10.1016/J.BIOMATERIALS.2009.09.063 PubMed Abstract | 10.1016/J.BIOMATERIALS.2009.09.063 | Google Scholar [DOI] [PubMed] [Google Scholar]
- Muzzarelli R., El Mehtedi M., Bottegoni C., Aquili A., Gigante A. (2015). Genipin-Crosslinked Chitosan Gels and Scaffolds for Tissue Engineering and Regeneration of Cartilage and Bone. Mar. Drugs 13, 7314–7338. 10.3390/MD13127068 PubMed Abstract | 10.3390/MD13127068 | Google Scholar [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nava M. M., Fedele R., Raimondi M. T. (2016). Computational Prediction of Strain-dependent Diffusion of Transcription Factors through the Cell Nucleus. Biomech. Model. Mechanobiol. 15, 983–993. 10.1007/S10237-015-0737-2 PubMed Abstract | 10.1007/S10237-015-0737-2 | Google Scholar [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen P. K., Neofytou E., Rhee J.-W., Wu J. C. (2016). Potential Strategies to Address the Major Clinical Barriers Facing Stem Cell Regenerative Therapy for Cardiovascular Disease. JAMA Cardiol. 1, 953–962. 10.1001/JAMACARDIO.2016.2750 PubMed Abstract | 10.1001/JAMACARDIO.2016.2750 | Google Scholar [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordberg R. C., Huebner P., Schuchard K. G., Mellor L. F., Shirwaiker R. A., Loboa E. G., et al. (2021). The Evaluation of a Multiphasic 3D ‐bioplotted Scaffold Seeded with Adipose Derived Stem Cells to Repair Osteochondral Defects in a Porcine Model. J. Biomed. Mater Res. 109, 2246–2258. 10.1002/JBM.B.34886 10.1002/JBM.B.34886 | Google Scholar [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nürnberger S., Schneider C., van Osch G. V. M., Keibl C., Rieder B., Monforte X., et al. (2019). Repopulation of an Auricular Cartilage Scaffold, AuriScaff, Perforated with an Enzyme Combination. Acta Biomater. 86, 207–222. 10.1016/j.actbio.2018.12.035 PubMed Abstract | 10.1016/j.actbio.2018.12.035 | Google Scholar [DOI] [PubMed] [Google Scholar]
- Ock S.-A., Lee Y.-M., Park J.-S., Shivakumar S. B., Moon S.-W., Sung N.-J., et al. (2016). Evaluation of Phenotypic, Functional and Molecular Characteristics of Porcine Mesenchymal Stromal/stem Cells Depending on Donor Age, Gender and Tissue Source. J. Veterinary Med. Sci. 78, 987–995. 10.1292/JVMS.15-0596 10.1292/JVMS.15-0596 | Google Scholar [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh S. H., Kim T. H., Im G. I., Lee J. H. (2010). Investigation of Pore Size Effect on Chondrogenic Differentiation of Adipose Stem Cells Using a Pore Size Gradient Scaffold. Biomacromolecules 11, 1948–1955. 10.1021/BM100199M PubMed Abstract | 10.1021/BM100199M | Google Scholar [DOI] [PubMed] [Google Scholar]
- Palumbo P., Lombardi F., Siragusa G., Cifone M., Cinque B., Giuliani M. (2018). Methods of Isolation, Characterization and Expansion of Human Adipose-Derived Stem Cells (ASCs): an Overview. Ijms 19, 1897. 10.3390/ijms19071897 PubMed Abstract | 10.3390/ijms19071897 | Google Scholar [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panadero J. A., Sencadas V., Silva S. C. M., Ribeiro C., Correia V., Gama F. M., et al. (2016). Mechanical Fatigue Performance of PCL-Chondroprogenitor Constructs after Cell Culture under Bioreactor Mechanical Stimulus. J. Biomed. Mat. Res. 104, 330–338. 10.1002/JBM.B.33386 PubMed Abstract | 10.1002/JBM.B.33386 | Google Scholar [DOI] [PubMed] [Google Scholar]
- Patrikoski M., Sivula J., Huhtala H., Helminen M., Salo F., Mannerström B., et al. (2014). Different Culture Conditions Modulate the Immunological Properties of Adipose Stem Cells. Stem Cells Transl. Med. 3, 1220–1230. 10.5966/SCTM.2013-0201 PubMed Abstract | 10.5966/SCTM.2013-0201 | Google Scholar [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prein C., Warmbold N., Farkas Z., Schieker M., Aszodi A., Clausen-Schaumann H. (2016). Structural and Mechanical Properties of the Proliferative Zone of the Developing Murine Growth Plate Cartilage Assessed by Atomic Force Microscopy. Matrix Biol. 50, 1–15. 10.1016/J.MATBIO.2015.10.001 PubMed Abstract | 10.1016/J.MATBIO.2015.10.001 | Google Scholar [DOI] [PubMed] [Google Scholar]
- Qiao Y., Liu X., Zhou X., Zhang H., Zhang W., Xiao W., et al. (2020). Gelatin Templated Polypeptide Co‐Cross‐Linked Hydrogel for Bone Regeneration. Adv. Healthc. Mat. 9, 1901239. 10.1002/ADHM.201901239 PubMed Abstract | 10.1002/ADHM.201901239 | Google Scholar [DOI] [PubMed] [Google Scholar]
- Rada T., Santos T. C., Marques A. P., Correlo V. M., Frias A. M., Castro A. G., et al. (2011). Osteogenic Differentiation of Two Distinct Subpopulations of Human Adipose-Derived Stem Cells: an In Vitro and In Vivo Study. J. Tissue Eng. Regen. Med. 6, 1–11. 10.1002/term.388 PubMed Abstract | 10.1002/term.388 | Google Scholar [DOI] [PubMed] [Google Scholar]
- Raposio E., Bertozzi N. (2017). How to Isolate a Ready-To-Use Adipose-Derived Stem Cells Pellet for Clinical Application. Eur. Rev. Med. Pharmacol. Sci. 21, 4252–4260. PubMed Abstract | Google Scholar [PubMed] [Google Scholar]
- Reppel L., Schiavi J., Charif N., Leger L., Yu H., Pinzano A., et al. (2015). Chondrogenic Induction of Mesenchymal Stromal/stem Cells from Wharton's Jelly Embedded in Alginate Hydrogel and without Added Growth Factor: an Alternative Stem Cell Source for Cartilage Tissue Engineering. Stem Cell Res. Ther. 6, 260. 10.1186/S13287-015-0263-2 PubMed Abstract | 10.1186/S13287-015-0263-2 | Google Scholar [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roddy E., DeBaun M. R., Daoud-Gray A., Yang Y. P., Gardner M. J. (2018). Treatment of Critical-Sized Bone Defects: Clinical and Tissue Engineering Perspectives. Eur. J. Orthop. Surg. Traumatol. 28, 351–362. 10.1007/S00590-017-2063-0 PubMed Abstract | 10.1007/S00590-017-2063-0 | Google Scholar [DOI] [PubMed] [Google Scholar]
- Rosen E. D., Spiegelman B. M. (2014). What We Talk about when We Talk about Fat. Cell 156, 20–44. 10.1016/J.CELL.2013.12.012 PubMed Abstract | 10.1016/J.CELL.2013.12.012 | Google Scholar [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruvinov E., Tavor Re'em T., Witte F., Cohen S. (2019). Articular Cartilage Regeneration Using Acellular Bioactive Affinity-Binding Alginate Hydrogel: A 6-month Study in a Mini-Pig Model of Osteochondral Defects. J. Orthop. Transl. 16, 40–52. 10.1016/J.JOT.2018.08.003 10.1016/J.JOT.2018.08.003 | Google Scholar [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadeghi A., Moztarzadeh F., Aghazadeh Mohandesi J. (2019). Investigating the Effect of Chitosan on Hydrophilicity and Bioactivity of Conductive Electrospun Composite Scaffold for Neural Tissue Engineering. Int. J. Biol. Macromol. 121, 625–632. 10.1016/J.IJBIOMAC.2018.10.022 PubMed Abstract | 10.1016/J.IJBIOMAC.2018.10.022 | Google Scholar [DOI] [PubMed] [Google Scholar]
- Sanchez-Adams J., Leddy H. A., McNulty A. L., O’Conor C. J., Guilak F. (2014). The Mechanobiology of Articular Cartilage: Bearing the Burden of Osteoarthritis. Curr. Rheumatol. Rep. 16, 1–9. 10.1007/S11926-014-0451-6 10.1007/S11926-014-0451-6 | Google Scholar [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez-Téllez D., Téllez-Jurado L., Rodríguez-Lorenzo L. (2017). Hydrogels for Cartilage Regeneration, from Polysaccharides to Hybrids. Polymers 9, 671. 10.3390/POLYM9120671 10.3390/POLYM9120671 | Google Scholar [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sándor G. B. (2012). Tissue Engineering of Bone: Clinical Observations with Adipose-Derived Stem Cells, Resorbable Scaffolds, and Growth Factors. Ann. Maxillofac. Surg. 2, 8–11. 10.4103/2231-0746.95308 PubMed Abstract | 10.4103/2231-0746.95308 | Google Scholar [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scalzone A., Ferreira A. M., Tonda-Turo C., Ciardelli G., Dalgarno K., Gentile P. (2019). The Interplay between Chondrocyte Spheroids and Mesenchymal Stem Cells Boosts Cartilage Regeneration within a 3D Natural-Based Hydrogel. Sci. Rep. 9, 14630. 10.1038/S41598-019-51070-7 PubMed Abstract | 10.1038/S41598-019-51070-7 | Google Scholar [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schek R. M., Taboas J. M., Segvich S. J., Hollister S. J., Krebsbach P. H. (2004). Engineered Osteochondral Grafts Using Biphasic Composite Solid Free-form Fabricated Scaffolds. Tissue Eng. 10, 1376–1385. 10.1089/TEN.2004.10.1376 PubMed Abstract | 10.1089/TEN.2004.10.1376 | Google Scholar [DOI] [PubMed] [Google Scholar]
- Schipper B. M., Marra K. G., Zhang W., Donnenberg A. D., Rubin J. P. (2008). Regional Anatomic and Age Effects on Cell Function of Human Adipose-Derived Stem Cells. Ann. Plast. Surg. 60, 538–544. 10.1097/SAP.0B013E3181723BBE PubMed Abstract | 10.1097/SAP.0B013E3181723BBE | Google Scholar [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert T., Lafont S., Beaurin G., Grisay G., Behets C., Gianello P., et al. (2013). Critical Size Bone Defect Reconstruction by an Autologous 3D Osteogenic-like Tissue Derived from Differentiated Adipose MSCs. Biomaterials 34, 4428–4438. 10.1016/J.BIOMATERIALS.2013.02.053 PubMed Abstract | 10.1016/J.BIOMATERIALS.2013.02.053 | Google Scholar [DOI] [PubMed] [Google Scholar]
- Scioli M. G., Bielli A., Gentile P., Cervelli V., Orlandi A. (2016). Combined Treatment with Platelet-Rich Plasma and Insulin Favours Chondrogenic and Osteogenic Differentiation of Human Adipose-Derived Stem Cells in Three-Dimensional Collagen Scaffolds. J. Tissue Eng. Regen. Med. 11, 2398–2410. 10.1002/term.2139 PubMed Abstract | 10.1002/term.2139 | Google Scholar [DOI] [PubMed] [Google Scholar]
- Seale P., Conroe H. M., Estall J., Kajimura S., Frontini A., Ishibashi J., et al. (2011). Prdm16 Determines the Thermogenic Program of Subcutaneous White Adipose Tissue in Mice. J. Clin. Invest. 121, 96–105. 10.1172/JCI44271 PubMed Abstract | 10.1172/JCI44271 | Google Scholar [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehy E. J., Mesallati T., Vinardell T., Kelly D. J. (2015). Engineering Cartilage or Endochondral Bone: a Comparison of Different Naturally Derived Hydrogels. Acta Biomater. 13, 245–253. 10.1016/J.ACTBIO.2014.11.031 PubMed Abstract | 10.1016/J.ACTBIO.2014.11.031 | Google Scholar [DOI] [PubMed] [Google Scholar]
- Sherwood J. K., Riley S. L., Palazzolo R., Brown S. C., Monkhouse D. C., Coates M., et al. (2002). A Three-Dimensional Osteochondral Composite Scaffold for Articular Cartilage Repair. Biomaterials 23, 4739–4751. 10.1016/S0142-9612(02)00223-5 PubMed Abstract | 10.1016/S0142-9612(02)00223-5 | Google Scholar [DOI] [PubMed] [Google Scholar]
- Shu W., Shu Y. T., Dai C. Y., Zhen Q. Z. (2012). Comparing the Biological Characteristics of Adipose Tissue-Derived Stem Cells of Different Persons. J. Cell. Biochem. 113, 2020–2026. 10.1002/JCB.24070 PubMed Abstract | 10.1002/JCB.24070 | Google Scholar [DOI] [PubMed] [Google Scholar]
- Silva G. A., Coutinho O. P., Ducheyne P., Reis R. L. (2007). Materials in Particulate Form for Tissue Engineering. 2. Applications in Bone. J. Tissue Eng. Regen. Med. 1, 97–109. 10.1002/TERM.1 PubMed Abstract | 10.1002/TERM.1 | Google Scholar [DOI] [PubMed] [Google Scholar]
- Song K., Li L., Yan X., Zhang Y., Li R., Wang Y., et al. (2016). Fabrication and Development of Artificial Osteochondral Constructs Based on Cancellous Bone/hydrogel Hybrid Scaffold. J. Mater Sci. Mater Med. 27, 1–12. 10.1007/s10856-016-5722-5 PubMed Abstract | 10.1007/s10856-016-5722-5 | Google Scholar [DOI] [PubMed] [Google Scholar]
- Supronowicz P., Gill E., Trujillo A., Thula T., Zhukauskas R., Ramos T., et al. (2011). Human Adipose-Derived Side Population Stem Cells Cultured on Demineralized Bone Matrix for Bone Tissue Engineering. Tissue Eng. Part A 17, 789–798. 10.1089/TEN.TEA.2010.0357 PubMed Abstract | 10.1089/TEN.TEA.2010.0357 | Google Scholar [DOI] [PubMed] [Google Scholar]
- Ter Huurne M., Schelbergen R., Blattes R., Blom A., De Munter W., Grevers L. C., et al. (2012). Antiinflammatory and Chondroprotective Effects of Intraarticular Injection of Adipose-Derived Stem Cells in Experimental Osteoarthritis. Arthritis & Rheumatism 64, 3604–3613. 10.1002/ART.34626 10.1002/ART.34626 | Google Scholar [DOI] [PubMed] [Google Scholar]
- Torres L., Dunlop D. D., Peterfy C., Guermazi A., Prasad P., Hayes K. W., et al. (2006). The Relationship between Specific Tissue Lesions and Pain Severity in Persons with Knee Osteoarthritis. Osteoarthr. Cartil. 14, 1033–1040. 10.1016/J.JOCA.2006.03.015 10.1016/J.JOCA.2006.03.015 | Google Scholar [DOI] [PubMed] [Google Scholar]
- Trujillo N., Popat K. (2014). Increased Adipogenic and Decreased Chondrogenic Differentiation of Adipose Derived Stem Cells on Nanowire Surfaces. Materials 7, 2605–2630. 10.3390/MA7042605 PubMed Abstract | 10.3390/MA7042605 | Google Scholar [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueyama H., Okano T., Orita K., Mamoto K., Ii M., Sobajima S., et al. (2020). Local Transplantation of Adipose-Derived Stem Cells Has a Significant Therapeutic Effect in a Mouse Model of Rheumatoid Arthritis. Sci. Rep. 10, 3076. 10.1038/S41598-020-60041-2 PubMed Abstract | 10.1038/S41598-020-60041-2 | Google Scholar [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varun T. K., Senani S., Jayapal N., Chikkerur J., Roy S., Tekulapally V. B., et al. (2017). Extraction of Chitosan and its Oligomers from Shrimp Shell Waste, Their Characterization and Antimicrobial Effect. Vet. world 10, 170–175. 10.14202/VETWORLD.2017.170-175 PubMed Abstract | 10.14202/VETWORLD.2017.170-175 | Google Scholar [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vériter S., André W., Aouassar N., Poirel H. A., Lafosse A., Docquier P.-L., et al. (2015). Human Adipose-Derived Mesenchymal Stem Cells in Cell Therapy: Safety and Feasibility in Different "Hospital Exemption" Clinical Applications. PLoS One 10, e0139566. 10.1371/JOURNAL.PONE.0139566 PubMed Abstract | 10.1371/JOURNAL.PONE.0139566 | Google Scholar [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Wenk E., Zhang X., Meinel L., Vunjak-Novakovic G., Kaplan D. L. (2009). Growth Factor Gradients via Microsphere Delivery in Biopolymer Scaffolds for Osteochondral Tissue Engineering. J. Control. Release 134, 81–90. 10.1016/J.JCONREL.2008.10.021 PubMed Abstract | 10.1016/J.JCONREL.2008.10.021 | Google Scholar [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weijers E. M., Van Den Broek L. J., Waaijman T., Van Hinsbergh V. W. M., Gibbs S., Koolwijk P. (2011). The Influence of Hypoxia and Fibrinogen Variants on the Expansion and Differentiation of Adipose Tissue-Derived Mesenchymal Stem Cells. Tissue Eng. Part A 17, 2675–2685. 10.1089/TEN.TEA.2010.0661 PubMed Abstract | 10.1089/TEN.TEA.2010.0661 | Google Scholar [DOI] [PubMed] [Google Scholar]
- Widhiyanto L., Utomo D. N., Perbowo A. P., Hernugrahanto K. D. (2020). Macroscopic and Histologic Evaluation of Cartilage Regeneration Treated Using Xenogenic Biodegradable Porous Sponge Cartilage Scaffold Composite Supplemented with Allogenic Adipose Derived Mesenchymal Stem Cells (ASCs) and Secretome: An In Vivo Experimental Study. J. Biomater. Appl. 35, 422–429. 10.1177/0885328220934938 PubMed Abstract | 10.1177/0885328220934938 | Google Scholar [DOI] [PubMed] [Google Scholar]
- Woodard L. N., Grunlan M. A. (2018). Hydrolytic Degradation and Erosion of Polyester Biomaterials. ACS Macro Lett. 7, 976–982. 10.1021/ACSMACROLETT.8B00424 PubMed Abstract | 10.1021/ACSMACROLETT.8B00424 | Google Scholar [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu G., Wu D., Lo J., Wang Y., Wu J., Lu S., et al. (2020). A Bioartificial Liver Support System Integrated with a DLM/GelMA-based Bioengineered Whole Liver for Prevention of Hepatic Encephalopathy via Enhanced Ammonia Reduction. Biomater. Sci. 8, 2814–2824. 10.1039/C9BM01879D PubMed Abstract | 10.1039/C9BM01879D | Google Scholar [DOI] [PubMed] [Google Scholar]
- Xu J., Wang W., Ludeman M., Cheng K., Hayami T., Lotz J. C., et al. (2008). Chondrogenic Differentiation of Human Mesenchymal Stem Cells in Three-Dimensional Alginate Gels. Tissue Eng. Part A 14, 667–680. 10.1089/TEA.2007.0272 PubMed Abstract | 10.1089/TEA.2007.0272 | Google Scholar [DOI] [PubMed] [Google Scholar]
- Xue R., Qian Y., Li L., Yao G., Yang L., Sun Y. (2017). Polycaprolactone Nanofiber Scaffold Enhances the Osteogenic Differentiation Potency of Various Human Tissue-Derived Mesenchymal Stem Cells. Stem Cell Res. Ther. 8, 148–149. 10.1186/S13287-017-0588-0/FIGURES/6 PubMed Abstract | 10.1186/S13287-017-0588-0/FIGURES/6 | Google Scholar [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamane S., Iwasaki N., Kasahara Y., Harada K., Majima T., Monde K., et al. (2007). Effect of Pore Size Onin Vitro Cartilage Formation Using Chitosan-Based Hyaluronic Acid Hybrid Polymer Fibers. J. Biomed. Mat. Res. 81A, 586–593. 10.1002/JBM.A.31095 10.1002/JBM.A.31095 | Google Scholar [DOI] [PubMed] [Google Scholar]
- Yang J., Zhang Y. S., Yue K., Khademhosseini A. (2017). Cell-laden Hydrogels for Osteochondral and Cartilage Tissue Engineering. Acta Biomater. 57, 1–25. 10.1016/J.ACTBIO.2017.01.036 PubMed Abstract | 10.1016/J.ACTBIO.2017.01.036 | Google Scholar [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao Y., Zeng L., Huang Y. (2016). The Enhancement of Chondrogenesis of ATDC5 Cells in RGD-Immobilized Microcavitary Alginate Hydrogels. J. Biomater. Appl. 31, 92–101. 10.1177/0885328216640397 PubMed Abstract | 10.1177/0885328216640397 | Google Scholar [DOI] [PubMed] [Google Scholar]
- Yin H., Wang Y., Sun X., Cui G., Sun Z., Chen P., et al. (2018). Functional Tissue-Engineered Microtissue Derived from Cartilage Extracellular Matrix for Articular Cartilage Regeneration. Acta Biomater. 77, 127–141. 10.1016/j.actbio.2018.07.031 PubMed Abstract | 10.1016/j.actbio.2018.07.031 | Google Scholar [DOI] [PubMed] [Google Scholar]
- Yoneno K., Ohno S., Tanimoto K., Honda K., Tanaka N., Doi T., et al. (2005). Multidifferentiation Potential of Mesenchymal Stem Cells in Three-Dimensional Collagen Gel Cultures. J. Biomed. Mat. Res. 75A, 733–741. 10.1002/JBM.A.30488 10.1002/JBM.A.30488 | Google Scholar [DOI] [PubMed] [Google Scholar]
- Yousefi A.-M., Hoque M. E., Prasad R. G. S. V., Uth N. (2015). Current Strategies in Multiphasic Scaffold Design for Osteochondral Tissue Engineering: A Review. J. Biomed. Mat. Res. 103, 2460–2481. 10.1002/JBM.A.35356 10.1002/JBM.A.35356 | Google Scholar [DOI] [PubMed] [Google Scholar]
- Yue K., Trujillo-de Santiago G., Alvarez M. M., Tamayol A., Annabi N., Khademhosseini A. (2015). Synthesis, Properties, and Biomedical Applications of Gelatin Methacryloyl (GelMA) Hydrogels. Biomaterials 73, 254–271. 10.1016/J.BIOMATERIALS.2015.08.045 PubMed Abstract | 10.1016/J.BIOMATERIALS.2015.08.045 | Google Scholar [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeltinger J., Sherwood J. K., Graham D. A., Müeller R., Griffith L. G. (2001). Effect of Pore Size and Void Fraction on Cellular Adhesion, Proliferation, and Matrix Deposition. Tissue Eng. 7, 557–572. 10.1089/107632701753213183 PubMed Abstract | 10.1089/107632701753213183 | Google Scholar [DOI] [PubMed] [Google Scholar]
- Zhang J., Huang X., Wang H., Liu X., Zhang T., Wang Y., et al. (2015). The Challenges and Promises of Allogeneic Mesenchymal Stem Cells for Use as a Cell-Based Therapy. Stem Cell Res. Ther. 6, 234. 10.1186/S13287-015-0240-9 PubMed Abstract | 10.1186/S13287-015-0240-9 | Google Scholar [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang K., He S., Yan S., Li G., Zhang D., Cui L., et al. (2016). Regeneration of Hyaline-like Cartilage and Subchondral Bone Simultaneously by Poly(l-Glutamic Acid) Based Osteochondral Scaffolds with Induced Autologous Adipose Derived Stem Cells. J. Mat. Chem. B 4, 2628–2645. 10.1039/C5TB02113H 10.1039/C5TB02113H | Google Scholar [DOI] [PubMed] [Google Scholar]
- Zhang X., Wang C., Liao M., Dai L., Tang Y., Zhang H., et al. (2019). Aligned Electrospun Cellulose Scaffolds Coated with rhBMP-2 for Both In Vitro and In Vivo Bone Tissue Engineering. Carbohydr. Polym. 213, 27–38. 10.1016/J.CARBPOL.2019.02.038 PubMed Abstract | 10.1016/J.CARBPOL.2019.02.038 | Google Scholar [DOI] [PubMed] [Google Scholar]
- Zhang X., Zheng Z., Liu P., Ma Y., Lin L., Lang N., et al. (2008). The Synergistic Effects of Microfracture, Perforated Decalcified Cortical Bone Matrix and Adenovirus-Bone Morphogenetic Protein-4 in Cartilage Defect Repair. Biomaterials 29, 4616–4629. 10.1016/J.BIOMATERIALS.2008.07.051 PubMed Abstract | 10.1016/J.BIOMATERIALS.2008.07.051 | Google Scholar [DOI] [PubMed] [Google Scholar]
- Zhao X., Lang Q., Yildirimer L., Lin Z. Y., Cui W., Annabi N., et al. (2016). Photocrosslinkable Gelatin Hydrogel for Epidermal Tissue Engineering. Adv. Healthc. Mat. 5, 108–118. 10.1002/ADHM.201500005 PubMed Abstract | 10.1002/ADHM.201500005 | Google Scholar [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou S., Cui Z., Urban J. P. G. (2004). Factors Influencing the Oxygen Concentration Gradient from the Synovial Surface of Articular Cartilage to the Cartilage-Bone Interface: A Modeling Study. Arthritis Rheum. 50, 3915–3924. 10.1002/art.20675 PubMed Abstract | 10.1002/art.20675 | Google Scholar [DOI] [PubMed] [Google Scholar]
- Žigon-Branc S., Markovic M., Van Hoorick J., Van Vlierberghe S., Dubruel P., Zerobin E., et al. (2019). Impact of Hydrogel Stiffness on Differentiation of Human Adipose-Derived Stem Cell Microspheroids. Tissue Eng. Part A 25, 1369–1380. 10.1089/ten.tea.2018.0237 PubMed Abstract | 10.1089/ten.tea.2018.0237 | Google Scholar [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuk P. A., Zhu M., Mizuno H., Huang J., Futrell J. W., Katz A. J., et al. (2001). Multilineage Cells from Human Adipose Tissue: Implications for Cell-Based Therapies. Tissue Eng. 7, 211–228. 10.1089/107632701300062859 PubMed Abstract | 10.1089/107632701300062859 | Google Scholar [DOI] [PubMed] [Google Scholar]