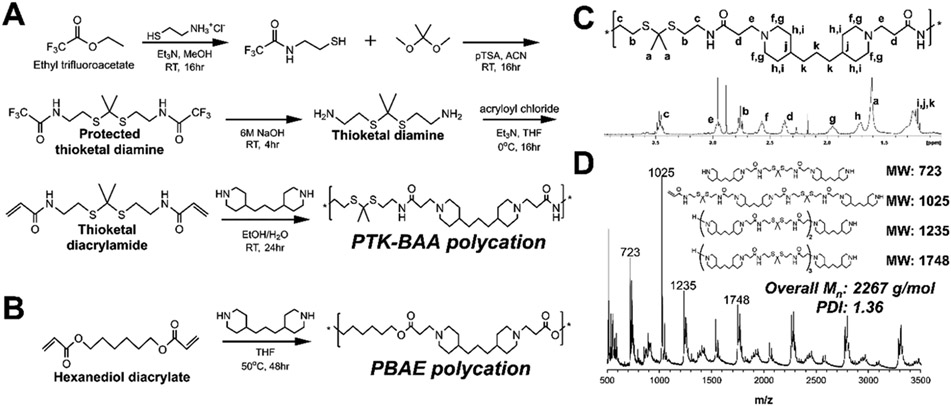
Figure 1.
Synthetic scheme for LbL-compatible polycations, (A) the oxidation-sensitive poly(thioketal β-amino amide) (PTK-BAA) and (B) the hydrolytically-degradable poly(β-amino ester) (PBAE). Both polycations are polymerized using Michael addition condensation and contain ionizable tertiary amines in the polymer backbone. However, the PTK-BAA features ROS-sensitive thioketal units and minimally-degradable amide linkers while the PBAE is degraded through the hydrolytically-labile ester bonds. Successful PTK-BAA polymer synthesis was confirmed by (C) 1H NMR spectroscopy and by (D) MALDI-TOF mass spectrometry. The presence of predicted polymerization products in the polymer bulk further validates the successful step-growth synthesis.