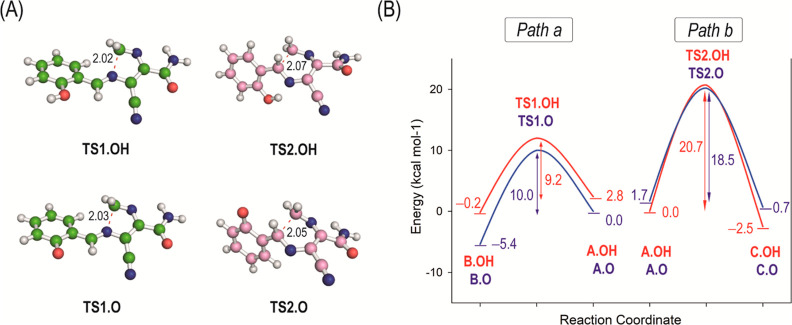
Figure 3.
(A) Geometries of the transition states TS1.OH, TS1.O, TS2.OH, and TS2.O obtained by computational studies. (B) Free energy profiles for the cyclization steps (paths a and b) to afford five- and six-membered derivatives substituted with a 2-phenol group. The two plausible protonation states of the phenol group were considered and are indicated with “.OH” (neutral form) and with “.O” (phenoxide form). For better comparison of the energy barriers of the cyclization processes, the energy of the most suitable conformer of A.OH and A.O that leads to the corresponding transition state is given.