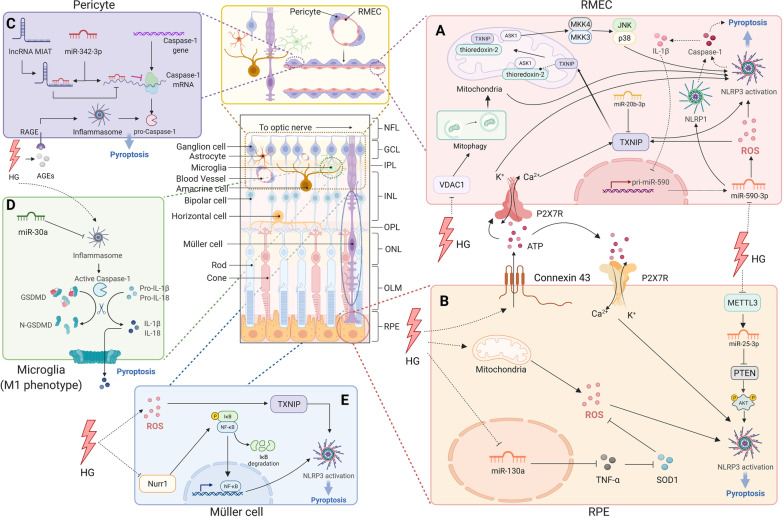
Fig. 3.
Molecular signaling pathway of pyroptosis in diabetic retinopathy. A Retinal microvascular endothelial cell (RMEC): increased extracellular ATP binds to P2X7R then activates NLRP3 inflammasome by causing K+ efflux and Ca2+ influx. TXNIP expression is induced through increased intracellular Ca2+ level, miR-590-3p/NOX4/ROS/TXNIP axis, or miR-20b-3p downregulation. After activation, the TXNIP shuttles to mitochondria, competes with apoptosis signal-regulating kinase 1 (ASK1), binds to NLRP3 and activates it; miR-590-3p also targets NLRP1. HG suppresses the voltage-dependent anion channel (VDAC1) expression, causing VDAC1/PINK1/Parkin-mediated mitophagy inhibition. Damaged mitochondria and mtROS accumulation results from impaired mitophagy-activated NLRP3 inflammasome. B Retinal pigment epithelium: hyperglycemia triggers the connexin43/ATP/P2X7R/Ca2+ influx pathway, mitochondrial ROS, miR-130a/TNF-α/SOD1/ROS pathway, or METTL3/miR-25-3p/PTEN/Akt/NLRP3 signaling cascade to activate NLRP3 inflammasome. C Pericyte: increased lncRNA MIAT competes with CASP1 mRNA for binding to miR-342-3p, blocking the CASP1 translation and CASP1-dependent pyroptosis. D Microglia (M1): MiR-30a downregulates NLRP3 expression. E Müller cell: hyperglycemia triggers the ROS/TXNIP axis to activate NLRP3 and downregulates the transcription factor nuclear receptor subfamily 4 group A member 2 (Nurr1) to suppress NLRP3 activation