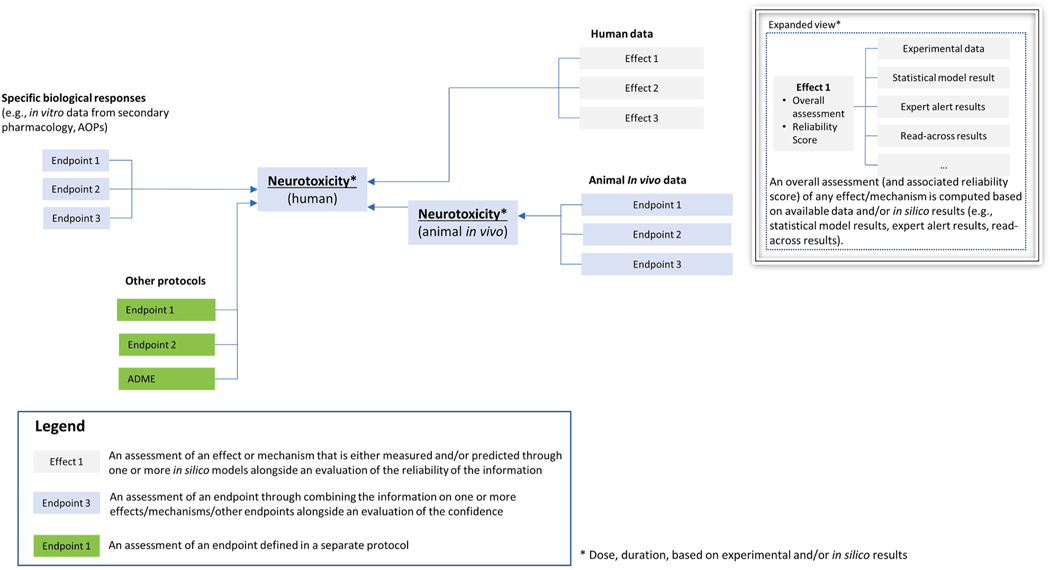
Fig. 1.
General outline of potential hazard assessment decision framework for neurotoxicity [59]. Data from different sources are integrated in the decision framework: in vitro data (e.g., biological responses from receptor-based assays), in vivo results, and human data. Other protocols (e.g., ADME or other organs) can contribute useful information (e.g., information on ADME processes is important to the interpretation of data from in vitro testing and complementary to, and supportive of, the prediction of neurotoxicity for human health hazard assessments; considerations on kinetic processes are necessary to relate in vitro findings to in vivo findings). Available information (e.g., environmental, drug, consumer, accidental) should also be used to supplement the protocol. Effects (predicted by in silico methods or measured experimentally) are combined for the assessment of a given endpoint. Combination of data is supplemented by an evaluation of the confidence that takes into consideration the quality of the information used to derive the assessment and the overall uncertainty in the assessment.