Abstract
Context.
The 5th edition of the World Health Organization classification of digestive system tumors discusses several advancements and developments in understanding the etiology, pathogenesis, and diagnosis of several digestive tract tumors.
Objective.
To provide a summary of the updates with a focus on neuroendocrine neoplasms, appendiceal tumors, and the molecular advances in tumors of the digestive system.
Data Sources.
English literature and personal experiences.
Conclusions.
Some of the particularly important updates in the 5th edition are the alterations made in the classification of neuroendocrine neoplasms, understanding of pathogenesis of appendiceal tumors and their precursor lesions, and the expanded role of molecular pathology in establishing an accurate diagnosis or predicting prognosis and response to treatment.
The 5th edition of the World Health Organization (WHO) classification of digestive system tumors published in 2019 discusses advancements that have been made in the understanding of etiology and pathogenesis of several digestive system tumors.
A particularly significant update discussed in the 5th edition pertains to the classification of neuroendocrine neoplasms (NENs), which can develop throughout the body. In the current WHO classification, neuroendocrine carcinomas (NECs) are all considered high-grade tumors. Previously, grade 1 and 2 tumors were regarded as neuroendocrine tumors (NETs) and grade 3 neoplasms as NECs. In the intervening years, grade 3 NETs were recognized1 and shown to be genetically unrelated to NECs. The new classification avoids the confusion between these 2 clinically and molecularly distinct entities.2 Another update noted in the latest edition relates to the understanding of appendiceal tumors and their precursor lesions along with standardization of terminology for appendiceal mucinous neoplasms.
The past decade has seen a growing understanding of molecular pathology of tumors. Knowledge of a tumor’s molecular characteristics plays a key role in the development of markers that aid in the diagnosis, management, and prediction of outcome of a neoplastic condition. While for certain types of tumors molecular analysis can be essential for making an accurate diagnosis, for other tumor types, specific tests are exploited to predict treatment response and prognosis. In the 5th edition, a greater emphasis has been placed on the molecular pathology of various types of digestive tumors.
This review aimed to provide a concise summary of selected updates from the 5th edition of the WHO classification mainly pertaining to NETs of the digestive system. An overview of each organ-specific NEN is also provided. The standardization of terminology for appendiceal tumors as well as the highlights of developments in molecular advances of tumors of the digestive system are also briefly discussed (see the Table for a summary of the selected updates).
NEUROENDOCRINE NEOPLASMS OF THE DIGESTIVE SYSTEM
General Characteristics of NENs
NENs can develop in almost all organs of the body. These tumors can vary widely in their clinical manifestations, morphology, genomic findings, and outcomes. Historically, NENs were classified as per their anatomic sites. This classification has resulted in divergent terminologies and criteria for the various organ systems. This issue was largely overcome in 2010, when the WHO published a uniform classification system for all NENs. The main feature of this new classification system is the distinction between well-differentiated NETs and poorly differentiated NECs. Although NETs and NECs are not closely related neoplasms, they share the expression of neuroendocrine markers.3-5 Clinical manifestations, epidemiologic data, histologic features, genetic evidence, and prognostic differences support the classification of NENs into NETs and NECs.5
| Summary of Selected Updates in 5th Edition of World Health Organization Classification of Digestive System Tumors |
|---|
| Summary of Selected Updates |
| G3 neuroendocrine tumor (NET) – New category formed for the grading of neuroendocrine neoplasms (NENs) |
| New Terminology for Mixed Neoplasms – Mixed neuroendocrine-non-neuroendocrine tumors – MiNENs (previously mixed adenoneuroendocrine carcinoma [MANEC]) |
| Terminology for appendiceal goblet cell carcinoma/carcinoid altered to appendiceal goblet cell adenocarcinoma |
| Category of hyperplastic and preneoplastic lesions has been abolished in the classification of neuroendocrine tumors (NETs) of the pancreas |
Clinical Differences: NETs and NECs
From a clinical perspective, the key distinction is between grade 3 (G3) NETs and NECs with respect to platinum-containing chemotherapy. Patients with NECs respond well to platinum-containing chemotherapy while patients with G3 NETs fail to respond to this therapy.6,7
Grading: NETs and NECs
The current grading system listed in the 5th edition is mainly based on the 2017 WHO classification of neuroendocrine neoplasms of the pancreas, for which outcome data are available. In 2017, this classification had formally introduced the concept of high-grade well-differentiated NETs.8
Prior to the release of this classification, the high-grade status (G3) was reserved only for poorly differentiated NECs. It is now understood that well-differentiated neuroendocrine neoplasms of various organs of the body can also demonstrate a proliferation rate in the G3 range such that this classification can be extended for NENs throughout the gastrointestinal tract.9
NETs are graded into G1, G2, and G3 based on the proliferation activity assessed by mitotic rate and/or Ki-67 proliferation index. The mitotic rates used for grading NETs are expressed as the number of mitoses/2 mm2, which is assessed by counting in 50 fields of 0.2 mm2. Although the mitotic rate yields an accurate assessment, it may be unreliable for small samples. To determine the Ki-67 proliferation index, at least 500 cells in the regions of highest labeling, known as “hotspots,” are counted. These areas are identified via scanning magnification. When areas with two varying proliferation indices are present in a particular sample, the area with higher proliferation index is selected for grading purposes.5,10
NECs are subtyped into small-cell NEC (SCNEC) and large-cell NEC (LCNEC). By definition, NECs are always high-grade neoplasms. Hence, as per the new WHO classification, NECs are not assigned any grade to avoid any confusion with neuroendocrine tumors in the G3 category.5
Morphologic Differences: NETs and NECs
Morphologically, NETs have organoid or nested architecture, uniform nuclear features, coarsely stippled chromatin, and minimal necrosis. NECs often grow in sheets and show a less nested architectural pattern. While small cell NECs have tightly packed fusiform nuclei with finely granular chromatin, LCNECs have more rounded, markedly atypical nuclei, and at times feature prominent nucleoli. Necrosis is abundant in NECs.5
In NETs, grade progression in morphologic features can be either noted in an individual tumor at the time of presentation or between varying anatomic locations of the disease (primary versus metastatic). If both high- and low-grade components are present in a single NET, this is strong evidence that the high-grade component is a well-differentiated neoplasm. In contrast, NECs seldom develop in combination with NETs. Most of the time, NECs develop in association with precursor lesions that lead to the formation of nonneuroendocrine carcinomas of the respective organs, such as colon adenomas or esophageal squamous dysplasia. Additionally, NECs may contain nonneuroendocrine elements, such as squamous cell carcinoma or adenocarcinoma.5,9 Neoplasms with substantial neuroendocrine and nonneuroendocrine components (>30% of either one) are classified as mixed neuroendocrine-nonneuroendocrine neoplasms (MiNENs). Such neoplasms only rarely contain a well-differentiated NET component in association with a nonneuroendocrine component.11
Genomic Differences: NETs and NECs
Growing genomic evidence indicates that NETs and NECs are unrelated neoplasms. Distinct genomic differences are especially noted in NENs of the pancreas, wherein gene mutations of NETs and NECs differ completely. In pancreatic NETs, the defining mutations are noted in the multiple endocrine neoplasia type 1 (MEN), death-domain-associated protein (DAXX), and alpha-thalassemia/intellectual disability syndrome X-linked (ATRX) genes.12 In NECs, these mutations are entirely absent and NECs instead show mutations in TP53, RB1, and less commonly in other carcinoma-related genes, such as KRAS, p16, Bcl-2, and Smad4/DPC4.13,14 In sporadic pancreatic NETs, germline mutations in the DNA repair genes, such as adenine DNA glycosylase activity of 14 human MutY homolog (MUTYH), cell cycle checkpoint kinase 2 (CHEK2), and breast cancer type 2 (BRCA2), can also be demonstrated.15 The mutation profile of pancreatic G3 NETs is similar to that of other well-differentiated neoplasms. This feature can be exploited in distinguishing high-grade NETs from NECs in challenging cases.16
Genomic comparisons of extrapancreatic NETs and NECs, mainly in gastrointestinal sites, are still emerging. It has been found that various gastrointestinal NECs frequently harbor mutations in the TP53 and RB1 genes similar to those in pancreatic lesions. Extrapancreatic NETs on the other hand often lack recurrent mutations. This proves to be a drawback in the genomic analysis of NETs for diagnostic purposes. Pancreatic and extrapancreatic NETs appear to share similarities with respect to chromatin remodeling pathways.5,17
NEUROENDOCRINE NEOPLASMS: HISTOLOGIC TYPES
Well-Differentiated NENs: NETs
NETs are well-differentiated epithelial neoplasms showing neuroendocrine differentiation by morphology and immunohistochemistry.3,5
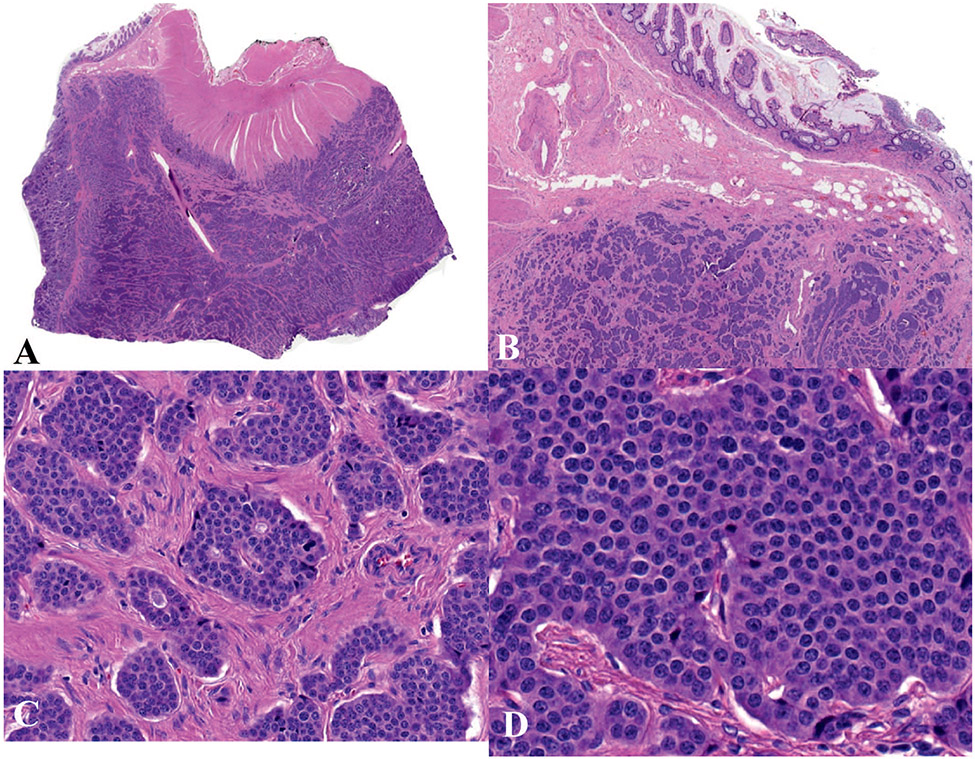
NETs can arise from any organ in the digestive system. Because of their well-differentiated nature, the cells forming NETs bear a strong resemblance to nonneoplastic neuroendocrine cells. Neuroendocrine differentiation is demonstrated by synaptophysin and chromogranin A immunohistochemistry. The morphologic features of NETs can be highly variable, with characteristic features in each specific organ. Generally, NETs display architectural patterns of cords, ribbons, and nests, but not uncommonly neoplastic cells can form tubules, especially in the pancreas, ileum, and a subset of appendiceal and duodenal NETs. The cells are typically round to oval with nuclei containing coarsely clumped, “salt and pepper” chromatin. Some NETs may show diffusely granular chromatin and some others may have prominent nucleoli. The cytoplasm is often intensely granular (Figure 1).4,5
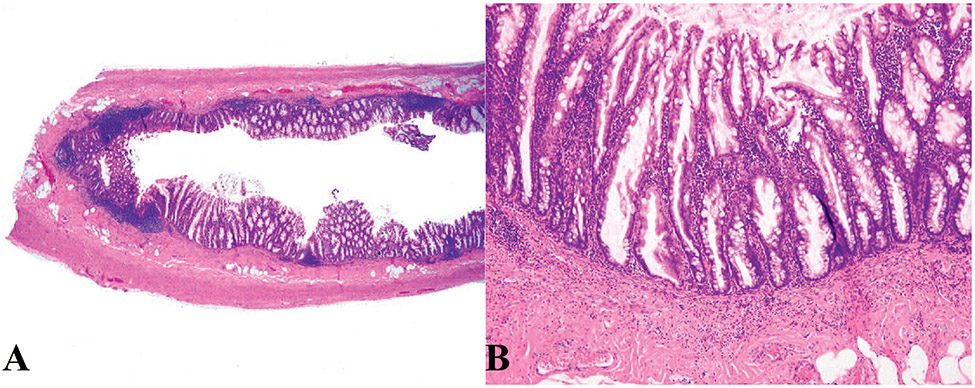
Figure 1.
Ileal neuroendocrine tumor, grade 1. A and b, Low and medium power view; the tumor displays architectural patterns of cords, ribbons, and nests. C and D, Higher-power view of (A) shows nests of neoplastic cells with round to oval nuclei containing coarsely clumped, “salt and pepper” chromatin (hematoxylin-eosin, original magnifications ×2 [A] and ×10 [B]; original magnification ×100 [C and D]).
In the 4th edition of WHO classification of digestive tumors, NETs were defined by a low proliferative rate (a mitotic rate <20 mitoses/2 mm2 and a Ki-67 proliferation index <20%). Although most NETs exhibit a low proliferative index, some cases, especially those that arise in the pancreas, can show a proliferative index as high as 70% to 80%. Therefore, Ki-67 proliferation index alone cannot be used to distinguish NETs from neuroendocrine carcinomas.5
Poorly Differentiated NENs: NECs
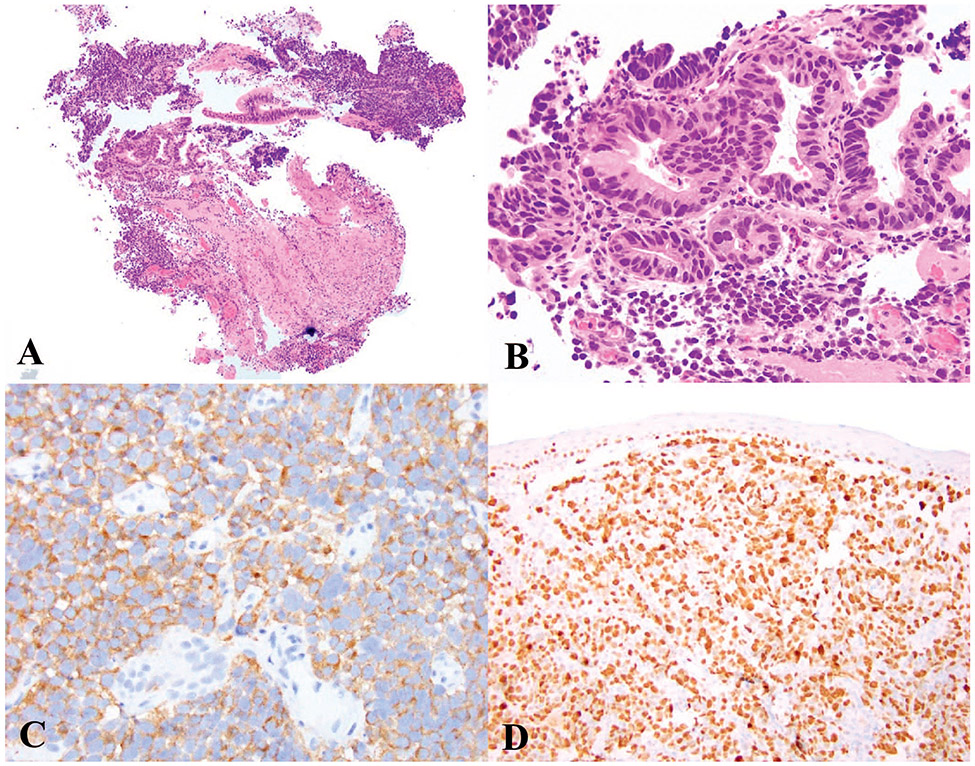
Similar to NETs, NECs can arise anywhere in the gastrointestinal tract. NECs are highly aggressive neoplasms and are usually at an advanced stage at the time of presentation. NECs are poorly differentiated epithelial neoplasms that show neuroendocrine differentiation by morphology and immunohistochemistry. Previously, the term NEC was also used for metastatic well-differentiated NETs but in the current classification, NEC only applies to poorly differentiated neoplasms. All NECs are considered high-grade neoplasms with more than 20 mitoses/2 mm2 and a Ki-67 proliferation index of higher than 20%. Occasionally, however, chemotherapy-treated NECs can have a Ki-67 proliferation index in the range of 20% to 50% and therefore, as above, proliferation index alone cannot be used to differentiate NECs from G3 NETs. Necrosis is often extensive in NECs. Neuroendocrine differentiation needs to be demonstrated by immunohistochemistry to confirm the diagnosis. Although the distinction can be challenging, NECs can be further subclassified as SCNEC or LCNEC. SCNEC display fusiform nuclei with finely granular chromatin, scant cytoplasm, and nuclear molding (Figure 2, A through D). LCNECs have large round vesicular nuclei, at times with prominent nucleoli, and moderate to abundant eosinophilic cytoplasm (Figure 3, A and B).3,5,18
Figure 2.
Small cell neuroendocrine carcinoma (SCNEC). A and B, SCNEC of the esophagus demonstrating solid sheets of small cells with high N:C ratio, fusiform nuclei, nuclear molding and scant cytoplasm arising in a background of high-grade dysplasia in Barrett esophagus. C, Immunohistochemical staining for synaptophysin shows diffuse staining with focal “dot-like” positivity in some cells. D, Ki-67 immunostaining, with a Ki-67 proliferation index of 90% (hematoxylin-eosin, original magnifications ×2 [A] and ×100 [B]; original magnifications ×200 [C] and ×100 [D]).
Figure 3.
Large cell neuroendocrine carcinoma (LCNEC). A and B, LCNEC composed of sheets of large cells with dispersed chromatin, prominent nucleoli, and moderate-to-abundant eosinophilic cytoplasm (hematoxylin-eosin, original magnifications ×10 [A] and ×200 [B]).
Mixed Neoplasms: MiNENs
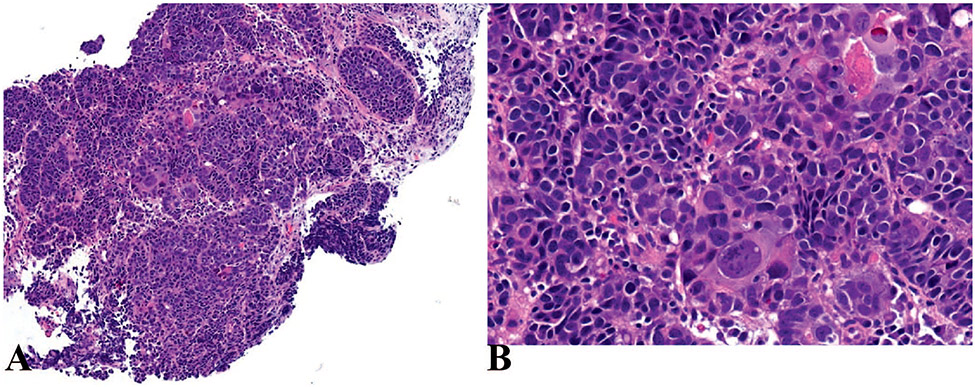
Neoplasms in which a neuroendocrine component is combined with a nonneuroendocrine component are now known as MiNEN. Previously, these mixed neoplasms were classified under the category of mixed adenoneuroendocrine carcinoma; however, it is now recognized that the nonneuroendocrine component is not necessarily adenocarcinoma and either one or both components may not be carcinomas. For a neoplasm to qualify as a MiNEN, both components should be morphologically and immunologically recognizable. The presence of a neuroendocrine component is confirmed by immunolabelling for synaptophysin and/or chromogranin A. By arbitrary convention, each component should constitute 30% or more of a neoplasm to be grouped as a MiNEN. Each component of a MiNEN should be graded individually (Figure 4).5,11
Figure 4.
Mixed neuroendocrine-non-neuroendocrine neoplasms (MiNEN). A, Low-power view; MiNEN of the gastric mucosa composed of adenocarcinoma and grade 1 neuroendocrine tumor (NET). B, Higher-power view of (A) shows the infiltrative glands of adenocarcinoma adjacent to grade 1 NET. C, Background of atrophic gastritis showing atrophic oxyntic glands and intestinal metaplasia. D, Immunohistochemical staining for synaptophysin shows diffuse and strong immunoreactivity in the neuroendocrine component whereas it is negative in the adenocarcinoma (hematoxylin-eosin, original magnifications ×10 [A] and ×100 [B and C]; original magnification ×200 [D]).
Because of possible response to platinum-based treatments, the presence of even a minor component of SCNEC should be mentioned in the diagnosis. The presence of a focal (<30%) neuroendocrine component does not change the diagnostic categorization but it may be mentioned in the report.5
NEUROENDOCRINE NEOPLASMS OF SPECIFIC ORGANS OF THE DIGESTIVE SYSTEM
Pancreatic Neuroendocrine Neoplasm
Among pancreatic tumors, neuroendocrine neoplasms account for approximately 2% to 5%. The incidence of pancreatic neuroendocrine neoplasms (PanNENs) is less than 1 case per 100 000 persons/year. PanNENs occur almost equally in both sexes, with the highest incidence observed in individuals between 30 and 60 years of age. The risk factors for PanNENs include obesity, diabetes, smoking, excess alcohol intake, and a family history of cancer.19 In surgically resected specimens, two-thirds of nonfunctioning pancreatic neuroendocrine tumors (PanNETs) are found in the head of the pancreas.5
PanNENs include well-differentiated NENs, known as NETs and poorly differentiated neoplasms, called NECs. Clinically, while PanNETs are slow-growing with a better survival rate, PanNECs grow rapidly with a poor survival rate.20
PanNETs can be further grouped into functioning and nonfunctioning neoplasms. Functioning PanNETs secrete hormones, resulting in clinical syndromes. Hence, these neoplasms are also known as syndromic PanNETs. Functioning PanNETs include gastrinomas, insulinomas, VIPo-mas, glucagonomas, and less commonly, tumors secreting ACTH, PTHrP, CCK, GHRH, and serotonin. Note that expression of various hormones by immunohistochemistry does not correlate well with function such that, for example, a neoplasm that expresses gastrin by immunostaining but that does not result in syndromic hypergastrinemia is not a gastrinoma.
Updated WHO Classification for Pancreatic NENs
One of the main alterations in the latest WHO classification is the formation of a new category called G3 PanNET. As per 2010 WHO classification, PanNENs displaying histologic features of PanNETs with a Ki-67 proliferation index more than 20% were classified as PanNECs. These tumors, which often manifest as liver metastases, are histologically bland, mitotically active, and lack the genetic abnormalities observed in the high-grade PanNENs. Behaviorally, these tumors are less aggressive than PanNECs, but have a worse prognosis than grade 1 and 2 PanNETs. In the 2017 updated WHO classification of NETs, these tumors were grouped under a novel category known as G3 PanNET.5,8,21
As per the updated classification, PanNENs categorized under G3 PanNET may show the following features:
Well-differentiated histologic pattern with a Ki-67 Proliferation rate more than 20% but less than 55%.
In general, primary G3 PanNETs have intact TP53 and RB1.
G3 PanNETs may contain low-grade components, or they may be found as metastases in patients with a prior G1 or G2 PanNET.
Another update in the 5th edition of the WHO classification is the terminology used for mixed neuroendocrine neoplasms of pancreatic and extrapancreatic neoplasms. Previously, these neoplasms were termed as mixed adenoneuroendocrine carcinoma. In the new classification, these neoplasms are known as MiNEN.5,11
In the 2017 WHO classification, the category of hyperplastic and preneoplastic lesions, which was included in the previous edition, was abolished. This alteration was introduced because PanNEN precursor changes have not been clearly identified in association with sporadic neoplasms and only described in the setting of multiple neuroendocrine neoplasia type 1, von Hippel-Lindau syndrome, and glucagon cell hyperplasia and neoplasia.22
Esophageal Neuroendocrine Neoplasms
Esophageal NENs are exceptionally rare, accounting for 0.04% to 1% of all gastroenteropancreatic NENs.23 They have a tendency to occur in the lower esophagus, often in association with Barrett mucosa and rarely with heterotopic gastric mucosa.24 Macroscopically, while esophageal NETs are small lesions, esophageal NECs are large, bulky, deeply infiltrative tumors that can be exophytic or ulcerated. Histologically, NETs can grow in insular or cribriform patterns. These tumors comprise medium-sized cells with a low N:C ratio, small ovoid nuclei, and dispersed chromatin containing small nucleoli. The expression of synaptophysin and chromogranin A is used to confirm neuroendocrine differentiation. NECs account for more than 90% of all esophageal NENs and are subtyped as SCNEC and LCNEC.25 Esophageal NECs typically show solid, rosette-like or palisading patterns. Extensive necrosis is often seen. Esophageal MiNENs are generally a combination of a poorly differentiated NEC and either squamous cell carcinoma or adenocarcinoma in a background of Barrett esophagus.5,26
Overall, patients with esophageal NETs have a good prognosis. Similar to those in other organs, esophageal NECs are associated with poor outcome.5
Gastric Neuroendocrine Neoplasms
The incidence of gastric NENs has been growing in recent years most likely due to the widespread use of endoscopy (increased detection). One study showed an increased incidence of gastric neuroendocrine tumors from 0.31 per 1 000 000 patients in 1975 to 4.85 in 2014.27
Gastric NETs have a site-specific distribution depending on the tumor subtype. For example, histamine-producing enterochromaffin-like (ECL) cell NETs arise in the corpus/fundus while somatostatin-expressing D-cell and gastrin-expressing G-cell NETs occur in the antrum. Serotonin-expressing enterochromaffin-cell NETs occur in both the antrum and corpus/fundus. Gastric NECs and MiNENs usually arise in the antrum or the cardiac regions.28
There are 3 types of gastric NETs. Type 1 ECL-cell NETs are the most common, have a female predominance and typically occur as multiple small polyps or nodules in the corpus or fundus. Type 1 ECL-cell NETs are associated with autoimmune chronic atrophic gastritis, which result in hypergastrinemia because of gastric hypoacidity. Type 2 ECL-cell NETs are rare and are seen in the setting of multiple endocrine neoplasia type 1 secondary to neoplastic hypergasterinemia as a result of gastrinoma, typically of the duodenum or pancreas. They tend to be multiple with a predilection for oxyntic mucosa. Type 3 NETs are not known to be associated with any specific etiology and they generally occur as solitary large lesions. Gastric NECs can present as large fungating masses that can deeply infiltrate the gastric wall.29
Patients with type 1 ECL-cell NETs have an excellent prognosis while those with type 3 ECL-cell NETs have the worst prognosis of all types. The prognosis of type 2 NETs is intermediate. Gastric NECs and MiNENs have a dismal prognosis.30
By histology, the histamine producing ECL-cell NETs show small microtobular and/or trabecular architecture without necrosis. These tumors consist of well-differentiated cells with abundant eosinophilic cytoplasm. They have monomorphic round nuclei that lack prominent nucleoli. Serotonin-expressing D-cell NETs display rounded nests of cells with peripheral palisading. The tumor cells are uniform and show intense eosinophilic cytoplasm. Gastrin-expressing G-cell NETs usually show a thin trabecular and gyriform pattern. Gastric NECs are composed of large, poorly formed trabeculae or sheets of poorly differentiated cells. Gastric SCNECs are composed of neoplastic cells with hyperchromatic nuclei and scant cytoplasm. In gastric LCNECs, there are large cells with vesicular nuclei with prominent nucleoli and eosinophilic cytoplasm. Gastric MiNENs are composed of areas of tubular, papillary, or mucinous adenocarcinoma and areas of G1 or G2 NET.5
Hepatic Neuroendocrine Neoplasms
Primary neuroendocrine neoplasms of the liver are very rare (much less common than metastases), accounting for 0.4% of resected hepatic primaries.31 Because of its rarity, a possibility of metastatic NEN should be excluded before considering a diagnosis of primary hepatic NEN.5 Histologically, hepatic NETs resemble those that arise in the upper gastrointestinal tract, pancreas, or rectum. They have nests and cords of uniform polygonal cells with hyperchromatic nuclei and coarsely clumped chromatin. The abundant cytoplasmic granularity seen in midgut NETs is absent in hepatic primary tumors and raises the possibility of metastasis from an occult primary. Hepatic NETs are generally WHO G1 or G2. There are no reports of hepatic G3 NETs in the literature.5,31
Both small and large cell hepatic NECs resemble those from extrahepatic organs and are typically mixed with a nonneuroendocrine component. Abundant necrosis and mitoses are often present.5 Hepatic MiNENs are more common than pure NECs. Most hepatic MiNENs contain a component of hepatocellular carcinoma, which may be the dominant component.5,32,33
Neuroendocrine Neoplasms of the Bile Ducts and Gallbladder
Biliary NENs are extremely rare, accounting for less than 1% of all NENs and are often incidentally detected during cholecystectomy.34 NECs are even rarer accounting for 4% of all malignant gallbladder neoplasms according to some studies.5,35 Both NETs and NECs have a slight female predominance and usually occur in the mid-seventh decade of life.34 NETs of the biliary tract tend to be associated with intraepithelial papillary neoplasms.36 The tumor cells are arranged in nests, trabeculae, and occasionally tubules. They have uniform round to oval nuclei and inconspicuous nucleoli. NECs of the biliary tract can be of small or large cell subtype and show morphologic features similar to those in other organs. Approximately one-third of biliary NECs are accompanied by an adenocarcinoma component. The NEC component dictates treatment and outcome.37
Small Intestinal and Ampullary Neuroendocrine Neoplasms
NENs of the small intestine and ampulla encompass duodenal, jejunal, and ileal epithelial neoplasms with neuroendocrine differentiation. The incidence of small intestinal NETs has been rising for the past 30 years. The majority of duodenal NETs (>95%) are located in the first or second part. The NETs located in the second part are mainly found in the ampullary region. Gangliocytic paraganglioma and somatostatin-expressing NET are essentially exclusive to the ampullary region. The distal ileum is the most common location for jejunoileal NETs. NECs of the small bowel are almost exclusive to the ampullary region.38
A minority of duodenal NETs are syndromic. Patients with multiple neuroendocrine neoplasia type 1 may develop multiple duodenal gastrinomas and a subset of ampullary somatostatin-expressing tumors occurs in the setting of neurofibromatosis type 1.39
Not uncommonly, patients with small intestine NETs present with locoregional lymph node and liver metastases (in up to 35% of cases according to large population-based studies).41 Despite this advanced presentation, these patients have a prolonged course. Studies assessing outcomes suggest a higher risk of long-term recurrence in patients with nodal metastases, mesenteric involvement, and lymphovascular or perineural invasion.40,41
Gastrin-expressing G-cell NETs, which tend to arise in duodenum, can show a trabecular pattern. Somatostatin-expressing D-cell NETs of the ampullary region are tubuloglandular, may contain psammoma bodies, and are associated with neurofibromatosis type 1. The serotonin-expressing enterochromaffin-cell NETs of the jejunoileal area may show peripheral palisading, and occasionally pseudogland formation. Gangliocytic paragangliomas are triphasic tumors composed of neuroendocrine, schwannian, and ganglion cell components. 5 Poorly differentiated NECs are high-grade carcinomas arranged in sheets, trabecular formations, or nests. Tumor cells are pleomorphic and may be of large or small cell subtype. An adenocarcinoma component may be present in MiNENs.
Colorectal Neuroendocrine Neoplasms
Colonic and rectal NENs have an incidence of 0.2 and 1.2 new cases per 100 000 persons/year, respectively.42 Colorectal NENs are usually silent or associated with nonspecific symptoms related to the mass, such as pain and hemorrhage. NECs and MiNENs may present with widespread metastases. Colonic NETs are typically larger than their small bowel, rectal, and appendiceal counterparts.43 Rectal NETs tend to be small; more than half are less than 1 cm in diameter.44 Histologic features of colorectal NETs and NECs are similar to those in other organs. MiNENs of the colorectum are composed of a poorly differentiated component and an adenocarcinoma component. MiNENs with a component of low-grade NET can rarely occur in the background of idiopathic inflammatory conditions.45,46
Anal Neuroendocrine Neoplasms
Anal neuroendocrine neoplasms account for a little more than 1% of all anal malignancies.47 Macroscopically, these tumors can present as ulcerated anal masses. Patients can complain of symptoms, such as anal pain, discomfort, or bleeding. Localized NETs of the anal canal have a good prognosis with a 10-year survival rate of 95%.48 An association between anal canal SCNEC and HIV infection has been documented49,50 as has an association with human papillomavirus.51 In the anal canal, it is particularly important to differentiate NECs and MiNENs from poorly differentiated (basaloid) squamous cell carcinoma, poorly differentiated adenocarcinoma, melanoma, basal cell carcinoma of perianal skin, and Merkel cell carcinoma. Immunohistochemistry can be helpful in confirming the diagnosis. Merkel cell carcinoma expresses neuroendocrine markers similar to SCNEC. However, the perinuclear dot-like pattern of cytokeratin 20 and negative TTF-1 support the diagnosis of Merkel cell carcinoma.52 Anal NECs may occasionally express TTF-1 such that it is not an ideal marker to differentiate primary anal NECs from metastatic NECs.53 Anal NETs usually have a nested or trabecular pattern. In most cases, anal NETs are G1 or G2.54 NECs show marked nuclear pleomorphism. MiNENs of the anal canal are rare and are often composed of NEC and adenocarcinoma components. MiNENs with SCNEC and squamous cell carcinoma components can also occur.5
Appendiceal Neuroendocrine Neoplasms
Appendiceal NETs typically arise in the tip of the appendix55 whereas appendiceal NECs do not have a predisposition toward a particular location and can occur in any part of the appendix.5 The majority of appendiceal NETs are discovered incidentally at appendectomy.56 Macroscopically, these neoplasms appear as yellowish, well-demarcated nodules. Appendiceal NETs consist of uniform polygonal tumor cells frequently arranged in large nests. They often show peripheral palisading and glandular formations similar to ileal NETs. Appendiceal NECs are exceedingly rare and are morphologically similar to colonic counterparts. MiNENs of the appendix are rare, and like their colonic counterparts, can display a combination of a NEC and adenocarcinoma. The term mixed adenoneuroendocrine carcinoma, which was previously used to describe goblet cell adenocarcinoma of the appendix, is no longer used. These tumors are now regarded as an adenocarcinoma subtype rather than a neuroendocrine neoplasm and staged accordingly.5,21
TUMORS OF THE APPENDIX
In the 5th edition of the WHO classification, appendiceal tumors have been broadly classified into the following categories:
Serrated lesions and polyps
Mucinous neoplasms
Adenocarcinomas
NENs
In this edition, the term “sessile serrated lesion” is preferred rather than “sessile serrated adenoma” or “sessile serrated polyp” mainly to reflect that not all these proliferations are polypoid in appearance; however, it is our opinion that these lesions behave like adenomas in that they are precursors, which would justify the original adenoma terminology, particularly in the colon.57 In a poll of our gastroenterology colleagues at Johns Hopkins Hospital in Baltimore, Maryland, 100% preferred retaining the term sessile serrated adenoma.
Goblet cell carcinoid/carcinoma has now been renamed to goblet cell adenocarcinoma. This alteration has been made with the logic that these tumors are composed predominantly of mucin-secreting cells and a minor component of neuroendocrine cells.
The main histopathologic features of different appendiceal tumors are discussed here.
Appendiceal Serrated Lesions and Polyps
Mucosal hyperplastic changes and polyps can certainly reflect reparative changes in postinflammatory settings in the appendix. However, based on genetic alterations (KRAS or BRAF mutations), some of the “hyperplastic” polyps are now known to be neoplastic. Some studies suggest that KRAS mutations are more biologically important in the Serrated lesions of the appendix than BRAF mutations and therefore the colonic serrated pathway of carcinogenesis might be less relevant in the appendix.58
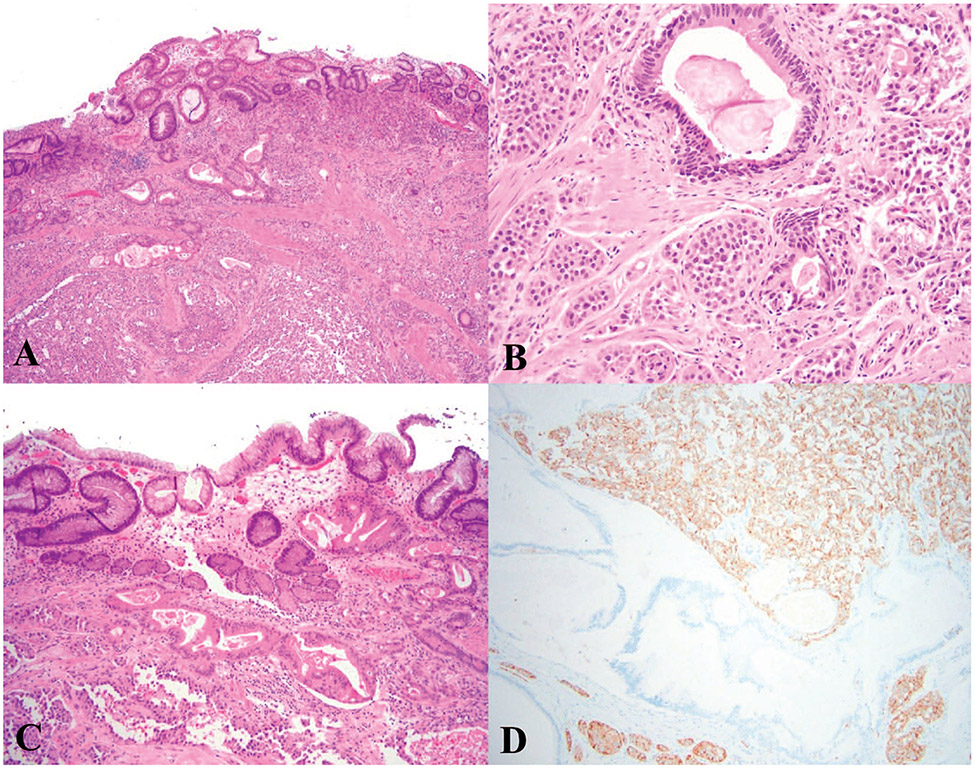
Similar to their colonic counterparts, hyperplastic polyps of the appendix show serration limited to their luminal aspects. In sessile serrated lesions without dysplasia, the mucosa demonstrates distorted crypts with crypt dilatation extending to the base of the crypts (Figure 5). In sessile serrated lesions with dysplasia, the serrated architecture is maintained. The dysplasia can resemble conventional adenoma-like dysplasia or traditional serrated adenoma-like dysplasia. The conventional adenoma-like dysplasia usually grows in a villous pattern with elongated, hyperchromatic nuclei with pseudostratification, increased mitoses, and apoptotic bodies. In traditional serrated adenoma-like dysplasia, there is complex serration with villous growth. The villi are lined by tall columnar cells with abundant eosinophilic cytoplasm.
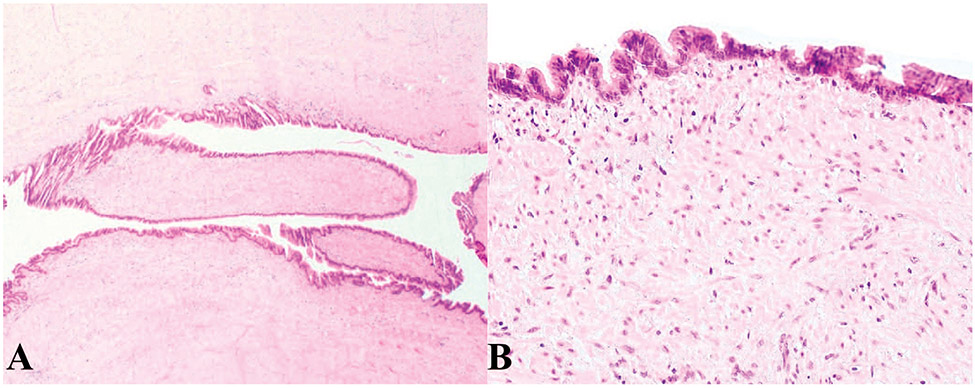
Figure 5.
Appendiceal sessile serrated lesion without dysplasia. A, Low-power view; the crypts are elongated with serrated appearance. B, High-power view shows the dilatation and lateral spread of the crypts at the base. The muscularis mucosae is intact (hematoxylin-eosin, original magnifications ×10 [A] and ×100 [B]).
In contrast to low-grade mucinous appendiceal neoplasms, which can also have serrated architecture, in sessile serrated adenomas/polyps/lesions, the muscularis mucosae and lamina propia are intact and the appendiceal mucosa retains its normal architecture.5 More importantly, and distinct from appendiceal mucinous neoplasms, rupture of sessile serrated adenoma/polyp/lesion is not associated with pseudomyxoma peritonei.
Appendiceal Mucinous Neoplasm
Appendiceal mucinous neoplasms are most common in women in their sixth decade of life. If the disease is restricted to the appendix, patients may present with acute appendicitis-like symptoms. Patients with disseminated disease may present with abdominal or ovarian masses or pseudomyxoma peritonei. Grossly, the appendix is dilated, containing luminal mucin. If the appendiceal wall is intact and unruptured, the serosa appears smooth. The presence of adhesions or extra-appendiceal mucin is concerning for an underlying rupture.
The nomenclature regarding appendiceal mucinous neoplasms is evolving with considerable controversy. The most important clinical implication of these neoplasms is the development of subsequent pseudomyxoma peritonei. The location of the neoplastic epithelium and associated mucin dictate the clinical consequences. A relatively recent consensus terminology has been established to address the controversial issues in definitions and terminology. By definition, appendiceal mucinous lesions with infiltrative invasion with associated dysplasia should be classified as “mucinous adenocarcinoma.” The term “cystadenoma” is no longer recommended and the term low-grade appendiceal mucinous neoplasm is preferred. Lesions without infiltrative invasion but high-grade cytology are termed high-grade appendiceal mucinous neoplasm.59
The classic pattern seen in appendiceal mucinous neoplasms is the replacement of normal appendiceal mucosa by a filiform villous mucinous epithelial proliferation. The cells in these neoplasms tend to have tall, cytoplasmic mucin vacuoles that compress the nucleus creating a hypermucinous appearance. In some cases, these tumors may show an undulating or scalloped pattern with columnar epithelial cells with nuclear pseudostratification and mild atypia resembling low-grade dysplasia in the colon. The submucosa and muscularis propria can show varying degrees of fibrosis and hyalinization (Figure 6).
Figure 6.
Low-grade appendiceal mucinous neoplasm. A, The mucosa is replaced by hypermucinous undulating epithelium. B, Higher-power view shows the epithelium lined by tall cells with low-grade cytological atypia and hyalinized, fibrotic submucosa with no significant inflammatory response (hematoxylin-eosin, original magnifications ×100 [A] and ×200 [B]).
Lesions associated with localized extra-appendiceal mucin devoid of epithelial cells are considered to be at low risk for recurrence or progression, occurring in less than 5% of cases. The presence of neoplastic epithelium within mucin increases the risk of recurrence and dissemination to 33% to 75% of the cases.60
Curiously, the 2019 classification avoided classifying low-grade appendiceal mucinous neoplasm and high-grade appendiceal mucinous neoplasm associated with extra-appendiceal spread as adenocarcinomas but referred to peritoneal disease as “dissemination to the peritoneal cavity” together with a table noting “peritoneal metastasis.” Indeed, the biology of such lesions is unusual and different from that of lesions in other parts of the tubular gut and lends itself to somewhat confusing terminology. However, the key point is that mucinous lesions confined to the appendix are cured by simple appendectomy. This confinement to the appendix includes tumors with pushing growth into the submucosa and muscularis propria without a desmoplastic response. Such extension is not associated with subsequent pseudomyxoma peritonei. Because of this, the staging was adjusted for these neoplasms. The T1 and T2 categories were eliminated and appendiceal mucinous neoplasms are staged as either Tis, T3, or T4. The T3 and T4 categories even include mucin without epithelial cells extending into the subserosa or serosa.
Close follow-up is recommended for patients with localized periappendiceal disease following initial surgery. Hyperthermic intraperitoneal chemotherapy following cytoreductive surgery may be used in patients with pseudomyxoma peritonei.61
Appendiceal Adenocarcinoma
Appendiceal adenocarcinomas can be mucinous, nonmucinous, or signet-ring subtype. KRAS and/or GNAS mutations are commonly found in mucinous subtype while BRAF mutations and microsatellite instability are rarely identified. High levels of microsatellite instability have been seen in nonmucinous adenocarcinomas. The pathogenesis of signet-ring adenocarcinomas is not known.62
Nonmucinous adenocarcinoma can show features identical to those of colorectal adenocarcinoma with irregular glands infiltrating the wall of the appendix, a desmoplastic stromal response, and dirty necrosis. Rarely, the morphology can resemble that of pancreatobiliary adenocarcinoma with glands lined by columnar cells with pale cytoplasm. If the tumor is composed of more than 50% extracellular mucin with floating glands, strips, or clusters of epithelial cells, it is considered a mucinous adenocarcinoma.
In general, the appendiceal primary tumors and the peritoneal tumor are graded concordantly and are graded in a three-tiered system. Low-grade mucinous appendiceal neoplasms are regarded as grade 1. Conventional mucinous adenocarcinoma is qualified as grade 2 and an adenocarcinoma with any number of signet-ring cells is considered grade 3.5,63
Nonmucinous adenocarcinomas of the appendix are graded based on a two-tiered grading system.
Appendiceal Goblet Cell Adenocarcinoma
Goblet cell adenocarcinoma, previously called “goblet cell carcinoid,” is a tumor composed of goblet-like mucinous cells, a variable number of endocrine cells, and Paneth-like cells.5 Goblet cell adenocarcinoma often involves the distal appendix. These tumors can have variable biological behavior from indolent to highly aggressive depending on the tumor grade.64 Low-grade tumors are typically arranged predominantly in tubules and usually involve the wall of the appendix circumferentially without eliciting a stromal reaction (Figure 7, A through D). Some tumor cells are not arranged in tubules and appear as clusters of cohesive goblet-like cells. There is mild nuclear atypia. Mitoses are infrequent.5 High-grade tumors display tumor cell infiltration as single mucinous or nonmucinous cells, complex anastomosing tubules, cribriform masses, sheets of tumor cells, large aggregates of goblet-like or signet-ring–like cells with high-grade cytological features, brisk mitoses, necrosis, and stromal reaction (Figure 7, E and F). For a tumor to be classified as goblet cell adenocarcinoma, a low-grade component must be present. Signet-ring cell adenocarcinoma, which is composed of signet ring cells with high-grade cytology, lacks the low-grade component. Although most goblet cell adenocarcinomas show focal expression of synaptophysin and chromogranin A, these stains are not required for the diagnosis.5
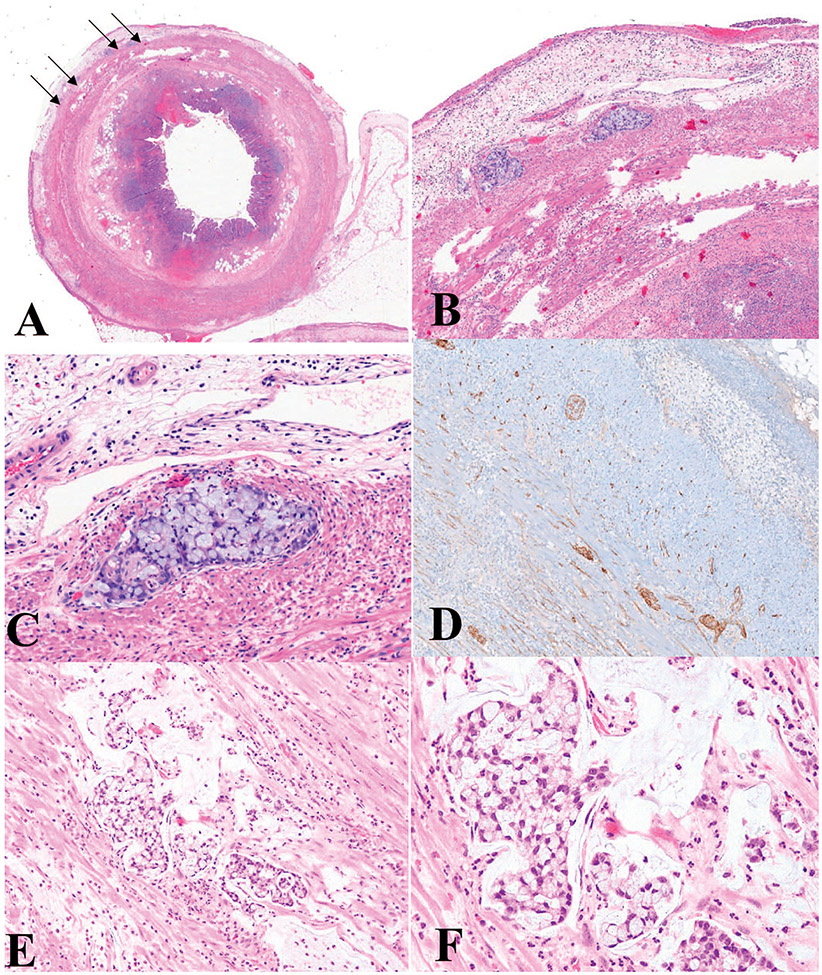
Figure 7.
Goblet cell adenocarcinoma, low grade (A–D). A, Low-power view; the tumor infiltrates the muscularis propria of the appendix as indicated by the arrows. B and C, High-power view of (A) shows clusters of cohesive goblet-like cells. D, Immunohistochemical stain for synaptophysin (not required for diagnosis) shows strong immunoreactivity in this case. Goblet cell adenocarcinoma, high grade (E, F). E, Low-power view; the tumor is infiltrative and shows complex anastomosing tubules. F, High-power view highlights the complex architectures with streaming of discohesive tumor cells with high-grade cytological features, in contrast to cohesive clusters of low-grade lesions (hematoxylin-eosin, original magnifications ×2 [A], ×100 [B, E], ×200 [C, F]; original magnification ×100 [D]).
A recent study, the results of which were used to create the classification and grading system, suggested a three-tiered system for grading goblet cell adenocarcinomas based on the proportion of tubular or clustered growth pattern. In this system, tumors with a tubular or clustered growth pattern more than 75% are considered low-grade, those with 50% to 75% tubular or clustered growth pattern are intermediate grade, and if the tumor shows less than 50% tubular or clustered growth pattern, it is qualified as high-grade.64 This scheme resulted in good stratification of patients in terms of survival and prognosis. Patients with intermediate grade (grade 2) tumors have a lower overall survival (60–80 months) in comparison to the lower grade (grade 1) tumors (84–204 months), and a better overall survival than high-grade (grade 3) tumors (29–45 months). A similar three-tiered system was used by Tang et al65 in the past.
MOLECULAR ADVANCES
Microsatellite instability
The DNA mismatch repair (MMR) apparatus comprises four proteins (MLH1, PMS2, MSH2, and MSH6) partnered as heterodimers. These proteins are responsible for recognition and repair of misincorporated bases during DNA replication. MLH1 partners with PMS2 and MSH2 partners with MSH6 to recognize the errors. In each heterodimer, one protein is dominant and is expressed independent of the partner status and the other is dependent wherein the protein is not expressed in the absence of the dominant partner. MMR deficiency can either occur as a result of germline mutation in MLH1, MSH2, MSH6, or PMS2 genes in Lynch syndrome or sporadically, due to MLH1 promoter methylation that leads to absent expression of MLH1 and PMS2.66
Lynch syndrome is an autosomal-dominant hereditary cancer susceptibility syndrome. This syndrome is characterized by an increased risk of several types of cancers including colorectal cancer (CRC) and endometrial cancer. Lynch syndrome–associated tumors are deficient in DNA MMR proteins. The diagnosis of Lynch syndrome is of paramount importance because it can significantly affect the management of the patient and allows for identification of at-risk family members.67
In addition to detecting Lynch syndrome, testing for MMR function is increasingly used to select adjuvant therapy in CRC, especially in the case of stage II disease. Colorectal cancers with deficient MMR function and wild-type BRAF are associated with a better stage for stage survival relative to those with intact MMR function. Additionally, it has been shown by various studies that regardless of BRAF status, patients with MMR-deficient tumors do not benefit from 5-fluorouracil–based chemotherapy. The presence of microsatellite instability (MSI) is also important in the setting of cancer immunotherapy. Recent studies have reported a significant response to programmed death ligand-1 inhibitors in MSI cancers, especially in patients who failed conventional therapy. Therefore, assessment of MMR function, either by immunohistochemistry (IHC) or MSI testing, is increasingly used to inform clinical decision-making regarding adjuvant chemotherapy or cancer immunotherapy, in particular for patients with stage II cancer or patients who did not improve while taking conventional therapy.68,69
Mismatch repair protein immunohistochemical staining is the most frequently used and convenient screening method. Antibodies to the 4 DNA MMR proteins are commercially available. The diagnosis of MMR deficiency is made when there is a complete absence of nuclear expression of one or more proteins, with intact expression in adjacent nonneoplastic tissues. Occasionally, however, IHC can exhibit equivocal results and potentially lead to misdiagnosis. A recent study showed that a weak or indeterminate MMR IHC expression might be an indicator of MSI-high or germline mutations.70
MSI testing by polymerase chain reaction is another screening test that can be used for diagnostic purposes. Microsatellite repeats are short-nucleotide repeats that are especially prone to inaccurate DNA replication. In MMR-deficient tumors, these replication mistakes are not corrected, leading to expansion or contraction of these repeats or MSI.
PREDICTIVE OR THERAPEUTIC MARKERS FOR GASTROINTESTINAL MALIGNANCIES – ASSORTED SITES
Predictive or therapeutic markers are indicators that predict response or a lack thereof to a specific therapy. A few important predictive markers that are currently used in gastrointestinal malignancies are as follows.
Human epidermal growth receptor 2 (HER2) testing has become the standard of care in advanced gastroesophageal junction, lower esophageal adenocarcinoma, and gastric carcinomas. A recent study showed that patients whose esophageal adenocarcinomas have human epidermal growth receptor 2 overexpression have better survival71 with neoadjuvant chemoradiation therapy, even using a protocol that includes carboxyplatin and paclitaxel rather than trastuzumab.72 Trastuzumab an antibody targeting the human epidermal growth factor receptor (EGFR) family member ERBB2 (human epidermal growth receptor 2), is now approved by the food and Drug Administration for the treatment of advanced or metastatic adenocarcinoma of esophagogastric junction in combination with other chemotherapeutic agents. The 2 common methods approved by the Food and Drug Administration to assess human epidermal growth receptor 2 status are IHC to detect protein overexpression and fluorescence in situ hybridization to evaluate ERBB2 gene amplification.7
There is no standardized algorithmic guideline regarding ERBB2 testing. Some organizations recommend both IHC and in situ hybridization, whereas others recommend performing fluorescence in situ hybridization only when IHC findings are equivocal.
Gastric cancers with Epstein-Barr virus positivity or mismatch repair deficiency (MSI-high) are associated with a better prognosis than others. Epstein-Barr virus–positive gastric cancers, seen more commonly in males, are associated with lymphoid stroma, a syncytial growth pattern and intraepithelial lymphocytes.73
A subset of MMR-deficient tumors has similar histologic features. Because of their prognostic significance, EBER in situ hybridization and MMR testing are recommended in cases with the abovementioned histologic features.74,75
The role of immunotherapy and routine evaluation of microsatellite instability and PD-L1 overexpression as potential biomarkers for prediction of response to immune checkpoint inhibitors in gastroesophageal adenocarcinomas are currently being investigated in clinical trials.76,77
In small intestine adenocarcinoma, MSI determination is required to evaluate for the possibility of hereditary causes and selection of patients who might benefit from immunotherapy.69,78 Ampullary undifferentiated carcinomas rarely show a distinct rhabdoid morphology in which the cells are discohesive within a myxoid matrix. In these tumors loss of nuclear immunostaining for SMARCB1 (INI1) is characteristic.79
RAS oncogenes have biological significance in CRC. KRAS and NRAS mutations, in particular, are associated with resistance to anti-EGFR (HER1) therapy. Approximately 50% of CRC harbor a clinically relevant RAS mutation and therefore should not be treated with anti-EGFR antibody therapy. RAS mutational analysis helps to select patients with metastatic CRC for anti-EGFR therapy.80,81
Although BRAF mutations are only found in 7% to 10% of patients with CRC, their presence conveys a very poor prognosis with a median survival of less than 12 months. The presence of BRAF mutations is also used to exclude Lynch syndrome. RAS and BRAF mutations are mutually exclusive. The role of anti-EGFR therapy remains controversial in BRAF-mutated CRCs; however, it has been shown by several studies that patients with BRAF mutations may not benefit from anti-EGFR therapy.82,83
P16 and human papillomavirus testing is now recommended for anal squamous lesions. It is now suggested that human papillomavirus plays an important role in the etiology of anal squamous lesions similar to cervical cancer.5
SUMMARY
There have been major advances in our understanding of the development of many gastrointestinal neoplasms since the publication of the 4th edition of the WHO classification of digestive system tumors. We have seen new insights into the biology and management of digestive system neoplasms and more targeted and effective therapies are being investigated. The 2019 WHO classification provides updated diagnostic categories and criteria, together with biological and clinical correlates. Here, we aimed to summarize the most important changes since the 2010 edition with a detailed focus on neuroendocrine neoplasms, appendiceal tumors, and molecular advances in the field.
Footnotes
The authors have no relevant financial interest in the products or companies described in this article.
References
- 1.Basturk O, Yang Z, Tang LH, et al. The high-grade (WHO G3) pancreatic neuroendocrine tumor category is morphologically and biologically heterogenous and includes both well differentiated and poorly differentiated neoplasms. Am J Surg Pathol. 2015;39(5):683–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nagtegaal ID, Odze RD, Klimstra D, et al. ; for the WHO Classification of Tumours Editorial Board. The 2019 WHO classification of tumours of the digestive system. Histopathology. 2020;76(2):182–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim JY, Hong S. Recent updates on neuroendocrine tumors from the gastrointestinal and pancreatobiliary tracts. Arch Pathol Lab Med. 2016;140(5):437–448. [DOI] [PubMed] [Google Scholar]
- 4.Rindi G, Klimstra DS, Abedi-Ardekani B, et al. A common classification framework for neuroendocrine neoplasms: an international agency for research on cancer (IARC) and world health organization (WHO) expert consensus proposal. Mod Pathol. 2018;31(12):1770–1786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lokuhetty D, White V, Watanabe R, Cree I. WHO classification of tumours of the digestive system. Lyon: International Agency for Research on Cancer; 2018. [Google Scholar]
- 6.Strosberg JR, Coppola D, Klimstra DS, et al. The NANETS consensus guidelines for the diagnosis and management of poorly differentiated (high-grade) extrapulmonary neuroendocrine carcinomas. Pancreas. 2010;39(6):799–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kunz PL, Mojtahed A, Fisher GA, et al. HER2 expression in gastric and gastroesophageal junction adenocarcinoma in a US population: clinicopathologic analysis with proposed approach to HER2 assessment. Appl Immunohistochem Mol Morphol. 2012;20(1):13–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Scoazec J, Couvelard A. Classification of pancreatic neuroendocrine tumours: Changes made in the 2017 WHO classification of tumours of endocrine organs and perspectives for the future [in French]. Ann Pathol. 2017;37(6):444–456. [DOI] [PubMed] [Google Scholar]
- 9.Tang LH, Untch BR, Reidy DL, et al. Well-differentiated neuroendocrine tumors with a morphologically apparent high-grade component: a pathway distinct from poorly differentiated neuroendocrine carcinomas. Clin Cancer Res. 2016;22(4):1011–1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van Velthuysen MF, Groen EJ, van der Noort V, van de Pol A, Tesselaar MET, Korse CM. Grading of neuroendocrine neoplasms: mitoses and ki-67 are both essential. Neuroendocrinology. 2014;100(2-3):221–227. [DOI] [PubMed] [Google Scholar]
- 11.La Rosa S, Sessa F, Uccella S. Mixed neuroendocrine-nonneuroendocrine neoplasms (MiNENs): unifying the concept of a heterogeneous group of neoplasms. Endocr Pathol. 2016;27(4):284–311. [DOI] [PubMed] [Google Scholar]
- 12.Jiao Y, Shi C, Edil BH, et al. DAXX/ATRX, MEN1, and mTOR pathway genes are frequently altered in pancreatic neuroendocrine tumors. Science. 2011;331(6021):1199–1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Konukiewitz B, Schlitter AM, Jesinghaus M, et al. Somatostatin receptor expression related to TP53 and RB1 alterations in pancreatic and extrapancreatic neuroendocrine neoplasms with a Ki67-index above 20. Mod Pathol. 2017;30(4):587–598. [DOI] [PubMed] [Google Scholar]
- 14.Yachida S, Vakiani E, White CM, et al. Small cell and large cell neuroendocrine carcinomas of the pancreas are genetically similar and distinct from well-differentiated pancreatic neuroendocrine tumors. Am J Surg Pathol. 2012;36(2):173–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Scarpa A, Chang DK, Nones K, et al. Whole-genome landscape of pancreatic neuroendocrine tumours. Nature. 2017;543(7643):65–71. [DOI] [PubMed] [Google Scholar]
- 16.Singhi AD, Klimstra DS. Well-differentiated pancreatic neuroendocrine tumours (PanNETs) and poorly differentiated pancreatic neuroendocrine carcinomas (PanNECs): Concepts, issues and a practical diagnostic approach to high-grade (G3) cases. Histopathology. 2018;72(1):168–177. [DOI] [PubMed] [Google Scholar]
- 17.Mafficini A, Scarpa A. Genetics and epigenetics of gastroenteropancreatic neuroendocrine neoplasms. Endocr Rev. 2019;40(2):506–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shia J, Tang LH, Weiser MR, et al. Is nonsmall cell type high-grade neuroendocrine carcinoma of the tubular gastrointestinal tract a distinct disease entity? Am J Surg Pathol. 2008;32(5):719–731. [DOI] [PubMed] [Google Scholar]
- 19.Kimura W, Kuroda A, Morioka Y. Clinical pathology of endocrine tumors of the pancreas. analysis of autopsy cases. Dig Dis Sci. 1991;36(7):933–942. [DOI] [PubMed] [Google Scholar]
- 20.Basturk O, Tang L, Hruban RH, et al. Poorly differentiated neuroendocrine carcinomas of the pancreas: a clinicopathologic analysis of 44 cases. Am J Surg Pathol. 2014;38(4):437–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lioyd RV, Osamura RY, Kloppel G. WHO classification of tumours of endocrine organs. Lyon: International Agency for Research on Cancer; 2010. [Google Scholar]
- 22.Esposito I, Segler A, Steiger K, Klöppel G. Pathology, genetics and precursors of human and experimental pancreatic neoplasms: an update. Pancreatology. 2015;15(6):598–610. [DOI] [PubMed] [Google Scholar]
- 23.Modlin IM, Shapiro MD, Kidd M. An analysis of rare carcinoid tumors: clarifying these clinical conundrums. World J Surg. 2005;29(1):92–101. [DOI] [PubMed] [Google Scholar]
- 24.Maru DM, Khurana H, Rashid A, et al. Retrospective study of clinicopathologic features and prognosis of high-grade neuroendocrine carcinoma of the esophagus. Am J Surg Pathol. 2008;32(9):1404–1411. [DOI] [PubMed] [Google Scholar]
- 25.Estrozi B, Bacchi CE. Neuroendocrine tumors involving the gastroenteropancreatic tract: a clinicopathological evaluation of 773 cases. Clinics (Sao Paulo). 2011;66(10):1671–1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hoang MP, Hobbs CM, Sobin LH, Albores-Saavedra J. Carcinoid tumor of the esophagus: a clinicopathologic study of four cases. Am J Surg Pathol. 2002;26(4):517–522. [DOI] [PubMed] [Google Scholar]
- 27.Yang Z, Wang W, Lu J, et al. Gastric neuroendocrine tumors (G-nets): incidence, prognosis and recent trend toward improved survival. Cell Physiol Biochem. 2018;45(1):389–396. [DOI] [PubMed] [Google Scholar]
- 28.La Rosa S, Vanoli A. Gastric neuroendocrine neoplasms and related precursor lesions. J Clin Pathol. 2014;67(11):938–948. [DOI] [PubMed] [Google Scholar]
- 29.Rindi G, Luinetti O, Cornaggia M, Capella C, Solcia E. Three subtypes of gastric argyrophil carcinoid and the gastric neuroendocrine carcinoma: a clinicopathologic study. Gastroenterology. 1993;104(4):994–1006. [DOI] [PubMed] [Google Scholar]
- 30.Li TT, Qiu F, Qian ZR, Wan J, Qi XK, Wu BY. Classification, clinicopathologic features and treatment of gastric neuroendocrine tumors. World J Gasteroenterol. 2014;20(1):118–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nomura Y, Nakashima O, Akiba J, et al. Clinicopathological features of neoplasms with neuroendocrine differentiation occurring in the liver. J Clin Pathol. 2017;70(7):563–570. [DOI] [PubMed] [Google Scholar]
- 32.Choi GH, Ann SY, Lee SI, Kim SB, Song IH. Collision tumor of hepatocellular carcinoma and neuroendocrine carcinoma involving the liver: case report and review of the literature. World J Gastroenterol. 2016;22(41):9229–9234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Okumura Y, Kohashi K, Wang H, et al. Combined primary hepatic neuroendocrine carcinoma and hepatocellular carcinoma with aggressive biological behavior (adverse clinical course): a case report. Pathol Res Pract. 2017;213(10):1322–1326. [DOI] [PubMed] [Google Scholar]
- 34.Zheng Z, Chen C, Li B, et al. Biliary neuroendocrine neoplasms: clinical profiles, management, and analysis of prognostic factors. Front Oncol. 2019;9:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Reid MD, Roa JC, Memis B, et al. Neuroendocrine neoplasms of the gallbladder. an immunohistochemical and clinicopathologic analysis of 29 cases [abstract 784]. Mod Pathol. 2017;30(S2):157–21028134903 [Google Scholar]
- 36.Adsay V, Jang K, Roa JC, et al. Intracholecystic papillary-tubular neoplasms (ICPN) of the gallbladder (neoplastic polyps, adenomas, and papillary neoplasms that are ≥1.0 cm): Clinicopathologic and immunohistochemical analysis of 123 cases. Am J Surg Pathol. 2012;36(9):1279–1301. [DOI] [PubMed] [Google Scholar]
- 37.Kanetkar AV, Patkar S, Khobragade KH, Ostwal V, Ramaswamy A, Goel M. Neuroendocrine carcinoma of gallbladder: a step beyond palliative therapy, experience of 25 cases. J Gastrointest Cancer. 2019;50(2):298–303. [DOI] [PubMed] [Google Scholar]
- 38.Terada T. Small cell carcinoma of the ileum that developed 10 years after total gastrectomy for gastric signet-ring cell carcinoma. Appl Immunohistochem Mol Morphol. 2012;20(6):618. [DOI] [PubMed] [Google Scholar]
- 39.O’Shea T, Druce M. When should genetic testing be performed in patients with neuroendocrine tumours? Rev Endocr Metab Disord. 2017;18(4):499–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Manguso N, Johnson J, Harit A, et al. Prognostic factors associated with outcomes in small bowel neuroendocrine tumors. Am Surg. 2017;83(10):1174–1178. [PubMed] [Google Scholar]
- 41.Frilling A, Modlin IM, Kidd M, et al. Recommendations for management of patients with neuroendocrine liver metastases. Lancet Oncol. 2014;15(1):8. [DOI] [PubMed] [Google Scholar]
- 42.Dasari A, Shen C, Halperin D, et al. Trends in the incidence, prevalence, and survival outcomes in patients with neuroendocrine tumors in the united states. JAMA Oncol. 2017;3(10):1335–1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Berardi RS. Carcinoid tumors of the colon (exclusive of the rectum): review of the literature. Dis Colon Rectum. 1972;15(5):383–391. [DOI] [PubMed] [Google Scholar]
- 44.Kojima M, Ikeda K, Saito N, et al. Neuroendocrine tumors of the large intestine: clinicopathological features and predictive factors of lymph node metastasis. Front Oncol. 2016;6:173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gaspar R, Santos-Antunes J, Marques M, et al. Mixed adenoneuroendocrine tumor of the rectum in an ulcerative colitis patient. GE Port J Gastroenterol. 2019;26(2):125–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wong M, Larson BK, Dhall D. Neuroendocrine proliferations in inflammatory bowel disease: differentiating neuroendocrine tumours from neuroendocrine cell micronests. Histopathology. 2019;74(3):415–423. [DOI] [PubMed] [Google Scholar]
- 47.Klas JV, Rothenberger DA, Wong WD, Madoff RD. Malignant tumors of the anal canal: the spectrum of disease, treatment, and outcomes. Cancer. 1999;85(8):1686–1693. [DOI] [PubMed] [Google Scholar]
- 48.Kim ST, Ha SY, Lee J, et al. The clinicopathologic features and treatment of 607 hindgut neuroendocrine tumor (NET) patients at a single institution. Medicine (Baltimore). 2016;95(19):e3534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nakahara H, Moriya Y, Shinkai T, Hirota T. Small cell carcinoma of the anus in a human HIV carrier: report of a case. Surg Today. 1993;23(1):85–88. [DOI] [PubMed] [Google Scholar]
- 50.Marcus DM, Edgar MA, Hawk NN, Sullivan PS, Stapleford LJ. Small cell carcinoma of the anus in the setting of prior squamous dysplasia and carcinoma in situ. J Gastrointest Oncol. 2013;4(2):E1–E4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cimino-Mathews A, Sharma R, Illei PB. Detection of human papillomavirus in small cell carcinomas of the anus and rectum. Am J Surg Pathol. 2012;36(7):1087–1092. [DOI] [PubMed] [Google Scholar]
- 52.Pasternak S, Carter MD, Ly TY, Doucette S, Walsh NM. Immunohistochemical profiles of different subsets of merkel cell carcinoma. Hum Pathol. 2018;82:232–238. [DOI] [PubMed] [Google Scholar]
- 53.Shia J. An update on tumors of the anal canal. Arch Pathol Lab Med. 2010;134(11):1601–1611. [DOI] [PubMed] [Google Scholar]
- 54.Gut P, Waligórska-Stachura J, Czarnywojtek A, et al. Hindgut neuroendocrine neoplasms – characteristics and prognosis. Arch Med Sci. 2017;13(6):1427–1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Stinner B, Rothmund M. Neuroendocrine tumours (carcinoids) of the appendix. Best Pract Res Clin Gastroenterol. 2005;19(5):729–738. [DOI] [PubMed] [Google Scholar]
- 56.Moris D, Tsilimigras DI, Vagios S, et al. Neuroendocrine neoplasms of the appendix: a review of the literature. Anticancer Res. 2018;38(2):601–611. [DOI] [PubMed] [Google Scholar]
- 57.Torlakovic E, Snover DC. Serrated adenomatous polyposis in humans. Gastroenterology. 1996;110(3):748–755. [DOI] [PubMed] [Google Scholar]
- 58.Pai RK, Hartman DJ, Gonzalo DH, et al. Serrated lesions of the appendix frequently harbor KRAS mutations and not BRAF mutations indicating a distinctly different serrated neoplastic pathway in the appendix. Hum Pathol. 2014;45(2):227–235. [DOI] [PubMed] [Google Scholar]
- 59.Carr NJ, Cecil TD, Mohamed F, et al. A consensus for classification and pathologic reporting of pseudomyxoma peritonei and associated appendiceal neoplasia: The results of the peritoneal surface oncology group international (PSOGI) modified Delphi process. Am J Surg Pathol. 2016;40(1):14–26. [DOI] [PubMed] [Google Scholar]
- 60.Yantiss RK, Shia J, Klimstra DS, Hahn HP, Odze RD, Misdraji J. Prognostic significance of localized extra-appendiceal mucin deposition in appendiceal mucinous neoplasms. Am J Surg Pathol. 2009;33(2):248–255. [DOI] [PubMed] [Google Scholar]
- 61.Shaib WL, Assi R, Shamseddine A, et al. Appendiceal mucinous neoplasms: diagnosis and management. Oncologist. 2017;22(9):1107–1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Liu X, Mody K, de Abreu FB, et al. Molecular profiling of appendiceal epithelial tumors using massively parallel sequencing to identify somatic mutations. Clin Chem. 2014;60(7):1004–1011. [DOI] [PubMed] [Google Scholar]
- 63.Davison JM, Choudry HA, Pingpank JF, et al. Clinicopathologic and molecular analysis of disseminated appendiceal mucinous neoplasms: identification of factors predicting survival and proposed criteria for a three-tiered assessment of tumor grade. Mod Pathol. 2014;27(11):1521–1539. [DOI] [PubMed] [Google Scholar]
- 64.Yozu M, Johncilla ME, Srivastava A, et al. Histologic and outcome study supports reclassifying appendiceal goblet cell carcinoids as goblet cell adenocarcinomas, and grading and staging similarly to colonic adenocarcinomas. Am J Surg Pathol. 2018;42(7):898–910. [DOI] [PubMed] [Google Scholar]
- 65.Tang LH, Shia J, Soslow RA, et al. Pathologic classification and clinical behavior of the spectrum of goblet cell carcinoid tumors of the appendix. Am J Surg Pathol. 2008;32(10):1429–1443. [DOI] [PubMed] [Google Scholar]
- 66.Roth RM, Hampel H, Arnold CA, Yearsley MM, Marsh WL, Frankel WL. A modified lynch syndrome screening algorithm in colon cancer: BRAF immunohistochemistry is efficacious and cost beneficial. Am J Clin Pathol. 2015;143(3):336–343. [DOI] [PubMed] [Google Scholar]
- 67.Walsh S. The pathology of Lynch syndrome. Diagnostic Histopathology. 2015;21(4):161–164. [Google Scholar]
- 68.Sinicrope FA. DNA mismatch repair and adjuvant chemotherapy in sporadic colon cancer. Nat Rev Clin Oncol. 2010;7(3):174–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Le DT, Uram JN, Wang H, et al. PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med. 2015;372(26):2509–2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sarode VR, Robinson L. Screening for lynch syndrome by immunohistochemistry of mismatch repair proteins: significance of indeterminate result and correlation with mutational studies. Arch Pathol Lab Med. 2019;143(10):1225–1233. [DOI] [PubMed] [Google Scholar]
- 71.Plum PS, Gebauer F, Krämer M, et al. HER2/neu (ERBB2) expression and gene amplification correlates with better survival in esophageal adenocarcinoma. BMC Cancer. 2019;19(1):38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.van Hagen P, Hulshof MC, van Lanschot JJ, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med. 2012;366(22):2074–2084. [DOI] [PubMed] [Google Scholar]
- 73.Iizasa H, Nanbo A, Nishikawa J, Jinushi M, Yoshiyama H. Epstein-Barr virus (EBV)-associated gastric carcinoma. Viruses. 2012;4(12):3420–3439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Huh CW, Jung DH, Kim H, et al. Clinicopathologic features of gastric carcinoma with lymphoid stroma in early gastric cancer. J Surg Oncol. 2016;114(6):769–772. [DOI] [PubMed] [Google Scholar]
- 75.Saito R, Abe H, Kunita A, Yamashita H, Seto Y, Fukayama M. Overexpression and gene amplification of PD-L1 in cancer cells and PD-L1+ immune cells in epstein-barr virus-associated gastric cancer: the prognostic implications. Mod Pathol. 2017;30(3):427–439. [DOI] [PubMed] [Google Scholar]
- 76.De Mello RA, Lordick F, Muro K, Janjigian YY. Current and future aspects of immunotherapy for esophageal and gastric malignancies. Am Soc Clin Oncol Educ Book. 2019(39):237–247. [DOI] [PubMed] [Google Scholar]
- 77.Bartley AN, Washington MK, Colasacco C, et al. HER2 testing and clinical decision making in gastroesophageal adenocarcinoma: guideline from the College of American Pathologists, American Society for Clinical Pathology, and the American Society of Clinical Oncology. J Clin Oncol. 2017;35(4):446–464. [DOI] [PubMed] [Google Scholar]
- 78.Jun S, Lee E, Kim M, et al. Lynch syndrome-related small intestinal adenocarcinomas. Oncotarget. 2017;8(13):21483–21500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Agaimy A, Rau TT, Hartmann A, Stoehr R. SMARCB1 (INI1)-negative rhabdoid carcinomas of the gastrointestinal tract: clinicopathologic and molecular study of a highly aggressive variant with literature review. Am J Surg Pathol. 2014;38(7):910–920. [DOI] [PubMed] [Google Scholar]
- 80.Bellizzi AM. Contributions of molecular analysis to the diagnosis and treatment of gastrointestinal neoplasms. Semin Diagn Pathol. 2013;30(4):329–361. [DOI] [PubMed] [Google Scholar]
- 81.Piawah S, Venook AP. Targeted therapy for colorectal cancer metastases: a review of current methods of molecularly targeted therapy and the use of tumor biomarkers in the treatment of metastatic colorectal cancer. Cancer. 2019;125(23):4139–4147. [DOI] [PubMed] [Google Scholar]
- 82.Luu L-J, Price TJ. BRAF mutation and its importance in colorectal cancer, advances in the molecular understanding of colorectal cancer. London: IntechOpen Limited; 2019. [Google Scholar]
- 83.Hsu H, Thiam TK, Lu Y, et al. Mutations of KRAS/NRAS/BRAF predict cetuximab resistance in metastatic colorectal cancer patients. Oncotarget. 2016;7(16):22257–22270. [DOI] [PMC free article] [PubMed] [Google Scholar]